Achivement
研究実績論文業績・学会発表
- 研究実績
-
論文業績・学会発表
論文業績・学会発表
- 研究実績
-
論文業績・学会発表
英文論文
- S, Wang ZX, Muro K, Morita S, Park YS, Zhang D, Yamada Y, Sakamoto J, Kim TW :Impact of prior chemotherapy with two different fluoropyrimidines on the efficacy of capecitabine plus irinotecan or FOLFIRI with or without bevacizumab in metastatic colorectal cancer: a post hoc analysis of the AXEPT study. Cancer Commun (Lond), 43(4):519-522,2023.4
- Narita Y, Muro K :Updated Immunotherapy for Gastric Cancer. J Clin Med, 12(7):2636,2023.4
- Matsubara Y, Masuishi T, Ogata T, Nakazawa T, Kato K, Nozawa K, Narita Y, Honda K, Bando H, Taniguchi H, Kadowaki S, Ando M, Tajika M, Muro K: Impact of omitting fluorouracil from FOLFIRI plus bevacizumab as second-line chemotherapy for patients with metastatic colorectal cancer. J Cancer Res Clin Oncol, 149(3):1123-1129,2023.3
- Chin K, Yamamoto S, Takahashi M, Kadowaki S, Kubota Y, Amanuma Y, Okada M, Kanda M, Kimura Y, Nogi Y, Arimitsu Y, Kitagawa Y:Effectiveness of taxanes following nivolumab in patients with advanced esophageal squamous cell carcinoma: a retrospective chart review of patients in ATTRACTION-3. Esophagus, 20(2):302-308,2023.4
- Watanabe J, Muro K, Shitara K, Yamazaki K, Shiozawa M, Ohori H, Takashima A, Yokota M, Makiyama A, Akazawa N, Ojima H, Yuasa Y, Miwa K, Yasui H, Oki E, Sato T, Naitoh T, Komatsu Y, Kato T, Hihara M, Soeda J, Misumi T, Yamamoto K, Akagi K, Ochiai A, Uetake H, Tsuchihara K, Yoshino T:Panitumumab vs Bevacizumab Added to Standard First-line Chemotherapy and Overall Survival Among Patients With RAS Wild-type, Left-Sided Metastatic Colorectal Cancer: A Randomized Clinical Trial. JAMA, 329(15):1271-1282,2023.4
- Chin K, Yamamoto S, Takahashi M, Kadowaki S, Kubota Y, Amanuma Y, Okada M, Kanda M, Kimura Y, Nogi Y, Arimitsu Y, Kitagawa Y :Effectiveness of taxanes following nivolumab in patients with advanced esophageal squamous cell carcinoma: a retrospective chart review of patients in ATTRACTION-3. Esophagus, 20(2):302-308,2023.4
- Ando M, Honda K, Hosoda W, Matsubara Y, Kumanishi R, Nakazawa T, Ogata T, Nakata A, Kodama H, Masuishi T, Narita Y, Taniguchi H, Kadowaki S, Muro K:Clinical outcomes of patients diagnosed with cancer of unknown primary or malignancy of undefined primary origin who were referred to a regional cancer center. Int J Clin Oncol, 28(5):644-653,2023.5
- Kuboki Y, Terazawa T, Masuishi T, Nakamura M, Watanabe J, Ojima H, Makiyama A, Kotaka M, Hara H, Kagawa Y, Sugimoto N, Kawakami H, Takashima A, Kajiwara T, Oki E, Sunakawa Y, Ishihara S, Taniguchi H, Nakajima TE, Morita S, Shirao K, Takenaka N, Ozawa D, Yoshino T:Trifluridine/tipiracil+bevacizumab (BEV) vs. fluoropyrimidine-irinotecan+BEV as second-line therapy for metastatic colorectal cancer: a randomised noninferiority trial. Br J Cancer, 128(10):1897-1905,2023.5
- Nakai C, Mimaki S, Matsushima K, Shinozaki E, Yamazaki K, Muro K, Yamaguchi K, Nishina T, Yuki S, Shitara K, Bando H, Suzuki Y, Akagi K, Nomura S, Fujii S, Sugiyama M, Nishida N, Mizokami M, Koh Y, Koshizaka T, Okada H, Abe Y, Ohtsu A, Yoshino T, Tsuchihara K:Regulation of MEK inhibitor selumetinib sensitivity by AKT phosphorylation in the novel BRAF L525R mutant. Int J Clin Oncol, 28(5):654-663,2023.5
- Inagaki C, Matoba R, Yuki S, Shiozawa M, Tsuji A, Inoue E, Muro K, Ichikawa W, Fujii M, Sunakawa Y:The BEETS (JACCRO CC-18) trial: an observational and translational study of BRAF-mutated metastatic colorectal cancer. Future Oncol, 19(17):1165-1174,2023.6
- Matsubara Y, Toriyama K, Kadowaki S, Ogata T, Nakazawa T, Kato K, Nozawa K, Narita Y, Honda K, Masuishi T, Bando H, Ando M, Tajika M, Oze I, Hosoda W, Muro K:The impact of combined PD-L1 positive score on clinical response to nivolumab in patients with advanced esophageal squamous cell carcinoma. Esophagus, 20(3):524-532,2023.7
- Ducreux M, Abou-Alfa GK, Bekaii-Saab T, Berlin J, Cervantes A, de Baere T, Eng C, Galle P, Gill S, Gruenberger T, Haustermans K, Lamarca A, Laurent-Puig P, Llovet JM, Lordick F, Macarulla T, Mukherji D, Muro K, Obermannova R, O'Connor JM, O'Reilly EM, Osterlund P, Philip P, Prager G, Ruiz-Garcia E, Sangro B, Seufferlein T, Tabernero J, Verslype C, Wasan H, Van Cutsem E:The management of hepatocellular carcinoma. Current expert opinion and recommendations derived from the 24th ESMO/World Congress on Gastrointestinal Cancer, Barcelona, 2022. ESMO Open, 8(3):101567,2023.6
- Kumanishi R, Kadowaki S, Mitani S, Matsushima T, Ogata T, Narita Y, Masuishi T, Bando H, Tajika M, Yasui H, Hara H, Muro K :Nivolumab versus irinotecan as third- or later-line treatment for advanced gastric cancer: a multi-center retrospective study. Int J Clin Oncol, 28(6):756-763,2023.6
- Habu T, Kumanishi R, Ogata T, Fujisawa T, Mishima S, Kotani D, Kadowaki S, Nakamura M, Hojo H, Fujiwara H, Kumagai S, Koyama S, Fujita T, Kinoshita T, Nishikawa H, Yano T, Tajika M, Muro K, Mitsunaga S, Kojima T, Bando H:Complete response to definitive chemoradiotherapy in unresectable locally advanced esophageal squamous cell carcinoma. Esophagus, 20(3):533-540,2023.7
- Kawabata R, Izawa N, Suzuki T, Nagahisa Y, Nishikawa K, Takahashi M, Nakamura M, Ishiguro A, Katsuya H, Hihara J, Manaka D, Negoro Y, Tsuji A, Takahashi T, Kochi M, Azuma M, Kadowaki S, Michimae H, Sunakawa Y, Ichikawa W, Fujii M:A Multicenter, Phase II Trial of Schedule Modification for Nab-Paclitaxel in Combination with Ramucirumab for Patients with Previously Treated Advanced Gastric or Gastroesophageal Junction Cancer: The B-RAX Trial (JACCRO GC-09). Target Oncol, 18(3):359-368,2023.5
- Shitara K, Di Bartolomeo M, Mandala M, Ryu MH, Caglevic C, Olesinski T, Chung HC, Muro K, Goekkurt E, McDermott RS, Mansoor W, Wainberg ZA, Shih CS, Kobie J, Nebozhyn M, Cristescu R, Cao ZA, Loboda A, Özgüroğlu M:Association between gene expression signatures and clinical outcomes of pembrolizumab versus paclitaxel in advanced gastric cancer: exploratory analysis from the randomized, controlled, phase III KEYNOTE-061 trial. J Immunother Cancer, 11(6):e006920,2023.6
- Ciardiello F, Bang YJ, Cervantes A, Dvorkin M, Lopez CD, Metges JP, Sánchez Ruiz A, Calvo M, Strickland AH, Kannourakis G, Muro K, Kawakami H, Wei J, Borg C, Zhu Z, Gupta N, Pelham RJ, Shen L:Efficacy and safety of maintenance therapy with pamiparib versus placebo for advanced gastric cancer responding to first-line platinum-based chemotherapy: Phase 2 study results. Cancer Med, 12(12):13145-13154,2023.6
- Izawa N, Masuishi T, Takahashi N, Shoji H, Yamamoto Y, Matsumoto T, Sugiyama K, Kajiwara T, Kawakami K, Aomatsu N, Kondoh C, Kawakami H, Takegawa N, Esaki T, Shimokawa M, Nishio K, Narita Y, Hara H, Sunakawa Y, Boku N, Moriwaki T, Eguchi Nakajima T, Muro K:A Phase II Trial of Trifluridine/Tipiracil in Combination with Cetuximab Rechallenge in Patients with RAS Wild-Type mCRC Refractory to Prior Anti-EGFR Antibodies: WJOG8916G Trial. Target Oncol, 18(3):369-381,2023.5
- Ogata T, Narita Y, Wainberg ZA, Van Cutsem E, Yamaguchi K, Piao Y, Zhao Y, Peterson PM, Wijayawardana SR, Abada P, Chatterjee A, Muro K:Exploratory Analysis of Patients With Gastric/Gastroesophageal Junction Adenocarcinoma With or Without Liver Metastasis From the Phase 3 RAINBOW Study. J Gastric Cancer, 23(2):289-302,2023.4
- Naito Y, Mishima S, Akagi K, Hayashi N, Hirasawa A, Hishiki T, Igarashi A, Ikeda M, Kadowaki S, Kajiyama H, Kato M, Kenmotsu H, Kodera Y, Komine K, Koyama T, Maeda O, Miyachi M, Nishihara H, Nishiyama H, Ohga S, Okamoto W, Oki E, Ono S, Sanada M, Sekine I, Takano T, Tao K, Terashima K, Tsuchihara K, Yatabe Y, Yoshino T, Baba E:Japanese Society of Medical Oncology/Japan Society of Clinical Oncology/Japanese Society of Pediatric Hematology/Oncology-led clinical recommendations on the diagnosis and use of tropomyosin receptor kinase inhibitors in adult and pediatric patients with neurotrophic receptor tyrosine kinase fusion-positive advanced solid tumors. Int J Clin Oncol, 28(7):827-840,2023.7
- Aoki Y, Nakamura Y, Denda T, Ohta T, Esaki T, Shiozawa M, Yamaguchi K, Yamazaki K, Sunakawa Y, Kato T, Okano N, Taniguchi H, Sato T, Oki E, Nishina T, Komatsu Y, Matsuhashi N, Goto M, Yasui H, Ohtsubo K, Moriwaki T, Takahashi N, Horita Y, Boku S, Wakabayashi M, Ikeno T, Mitani R, Yuasa M, Yoshino T:Clinical Validation of Plasma-Based Genotyping for RAS and BRAF V600E Mutation in Metastatic Colorectal Cancer: SCRUM-Japan GOZILA Substudy. JCO Precis Oncol, 7:e2200688,2023.6
- Le DT, Diaz LA Jr, Kim TW, Van Cutsem E, Geva R, Jäger D, Hara H, Burge M, O'Neil BH, Kavan P, Yoshino T, Guimbaud R, Taniguchi H, Élez E, Al-Batran SE, Boland PM, Cui Y, Leconte P, Marinello P, André T:Pembrolizumab for previously treated, microsatellite instability-high/mismatch repair-deficient advanced colorectal cancer: final analysis of KEYNOTE-164. Eur J Cancer, 186:185-195,2023.6
- Dasari A, Lonardi S, Garcia-Carbonero R, Elez E, Yoshino T, Sobrero A, Yao J, García-Alfonso P, Kocsis J, Cubillo Gracian A, Sartore-Bianchi A, Satoh T, Randrian V, Tomasek J, Chong G, Paulson AS, Masuishi T, Jones J, Csőszi T, Cremolini C, Ghiringhelli F, Shergill A, Hochster HS, Krauss J, Bassam A, Ducreux M, Elme A, Faugeras L, Kasper S, Van Cutsem E, Arnold D, Nanda S, Yang Z, Schelman WR, Kania M, Tabernero J, Eng C; FRESCO-2 Study Investigators:Fruquintinib versus placebo in patients with refractory metastatic colorectal cancer (FRESCO-2): an international, multicentre, randomised, double-blind, phase 3 study. Lancet, 402(10395):41-53,2023.6
- Yoshino T, Di Bartolomeo M, Raghav K, Masuishi T, Loupakis F, Kawakami H, Yamaguchi K, Nishina T, Wainberg Z, Elez E, Rodriguez J, Fakih M, Ciardiello F, Saxena K, Kobayashi K, Bako E, Okuda Y, Meinhardt G, Grothey A, Siena S; DESTINY-CRC01 investigators:Final results of DESTINY-CRC01 investigating trastuzumab deruxtecan in patients with HER2-expressing metastatic colorectal cancer. Nat Commun, 14(1):3332,2023.6
- Jenei K, Raymakers AJN, Bayle A, Berger-Thürmel K, Cherla A, Honda K, Jackson CCGA, Karikios D, Trapani D, Berry S, Gyawali B:Health technology assessment for cancer medicines across the G7 countries and Oceania: an international, cross-sectional study. Lancet Oncol, 24(6):624-635,2023.6
- Matsubara J, Mukai K, Kondo T, Yoshioka M, Kage H, Oda K, Kudo R, Ikeda S, Ebi H, Muro K, Hayashi R, Tokudome N, Yamamoto N, Muto M:First-Line Genomic Profiling in Previously Untreated Advanced Solid Tumors for Identification of Targeted Therapy Opportunities. JAMA Netw Open, 6(7):e2323336,2023.7
- Taniguchi H, Masuishi T, Ogata T, Ando M, Mizuno N, Muro K :First experience of a fully decentralized clinical trial: The dawn of a new era in oncology. Cancer Sci. 114(7):3050-3052,2023.7
- Tokunaga M, Kurokawa Y, Fukagawa T, Muro K, Shitara K, Kodera Y, Terashima M:Neoadjuvant chemotherapy for locally advanced gastric cancer in Japan: Consensus meeting at the 77th general meeting of the Japanese Society of Gastroenterological Surgery.Ann Gastroenterol Surg, 22;7(6):856-862, 2023.7
- Takahashi N, Hara H, Nagashima K, Hirata K, Masuishi T, Matsumoto T, Kawakami H, Yamazaki K, Hironaka S, Boku N, Muro K:Randomised phase II trial of trifluridine/tipiracil (FTD/TPI) plus ramucirumab (RAM) versus trifluridine/tipiracil for previously treated patients with advanced gastric or esophagogastric junction adenocarcinoma (RETRIEVE study, WJOG15822G). BMC Cancer, 23(1):726,2023.8
- Mishima S, Naito Y, Akagi K, Hayashi N, Hirasawa A, Hishiki T, Igarashi A, Ikeda M, Kadowaki S, Kajiyama H, Kato M, Kenmotsu H, Kodera Y, Komine K, Koyama T, Maeda O, Miyachi M, Nishihara H, Nishiyama H, Ohga S, Okamoto W, Oki E, Ono S, Sanada M, Sekine I, Takano T, Tao K, Terashima K, Tsuchihara K, Yatabe Y, Yoshino T, Baba E:Japanese Society of Medical Oncology/Japan Society of Clinical Oncology/Japanese Society of Pediatric Hematology/Oncology-led clinical recommendations on the diagnosis and use of immunotherapy in patients with high tumor mutational burden tumors. Int J Clin Oncol, 28(8):941-955,2023.8
- Booth CM, Sengar M, Goodman A, Wilson B, Aggarwal A, Berry S, Collingridge D, Denburg A, Eisenhauer EA, Ginsburg O, Goldstein D, Gunasekera S, Hammad N, Honda K, Jackson C, Karikios D, Knopf K, Koven R, Marini BL, Maskens D, Moraes FY, Mohyuddin GR, Poudyal BS, Pramesh CS, Roitberg F, Rubagumya F, Schott S, Sirohi B, Soto-Perez-de-Celis E, Sullivan R, Tannock IF, Trapani D, Tregear M, van der Graaf W, Vanderpuye V, Gyawali B:Common Sense Oncology: outcomes that matter. Lancet Oncol, 24(8):833-835,2023.8
- Yamamoto S, Wells K, Morita K, Tanigaki K, Muro K, Matsumoto M, Nakai H, Arai Y, Akizuki S, Takahashi K, Minamiguchi S, Fukuma S, Yanagita M:Severe TAFRO Syndrome Mimicking Hepatorenal Syndrome Successfully Treated with a Multidisciplinary Approach: A Case Report and Literature Review.Intern Med,62(18):2715-2724,2023.9
- Muro K, Shitara K, Yamaguchi K, Yoshikawa T, Satake H, Hara H, Sugimoto N, Machida N, Goto M, Kawakami H, Amagai K, Omuro Y, Esaki T, Hironaka S, Nishina T, Komatsu Y, Matsubara H, Shiratori S, Han S, Satoh T, Ohtsu A:Efficacy of Pembrolizumab Monotherapy in Japanese Patients with Advanced Gastric or Gastroesophageal Junction Cancer. J Gastrointest Cancer, 54(3):951-961,2023.9
- Kodama H, Masuishi T, Wakabayashi M, Nakata A, Kumanishi R, Nakazawa T, Ogata T, Matsubara Y, Honda K, Narita Y, Taniguchi H, Kadowaki S, Ando M, Muro K:Differential Efficacy of Targeted Monoclonal Antibodies in Left-Sided Colon and Rectal Metastatic Cancers. Clin Colorectal Cancer, 22(3):298-306,2023.9
- Yuki S, Yamazaki K, Sunakawa Y, Taniguchi H, Bando H, Shiozawa M, Nishina T, Yasui H, Kagawa Y, Takahashi N, Denda T, Esaki T, Kawakami H, Satake H, Takashima A, Matsuhashi N, Kato T, Asano C, Abe Y, Nomura S, Yoshino T:Role of plasma angiogenesis factors in the efficacy of first-line chemotherapy combined with biologics in RAS wild-type metastatic colorectal cancer: Results from the GI-SCREEN CRC-Ukit study. Cancer Med, 12(18):18702-18716,2023.9
- Masuishi T, Nagaoka S, Jin L, Yoshizawa K:Post-Marketing Safety Study of Ramucirumab Plus FOLFIRI: Analysis of Age and Initial Dose of Irinotecan in Patients with Metastatic Colorectal Cancer. Drugs Real World Outcomes, 10(3):405-413,2023.9
- Hayashi H, Sawada K, Tanaka H, Muro K, Hasebe T, Nakajima S, Okumura T, Fujiya M:The effect of heat-killed Lactobacillus brevis SBL88 on improving selective hepatic insulin resistance in non-alcoholic fatty liver disease mice without altering the gut microbiota.J Gastroenterol Hepatol, 38(10):1847-1854,2023.10
- Kojima Y, Mishiro-Sato E, Fujishita T, Satoh K, Kajino-Sakamoto R, Oze I, Nozawa K, Narita Y, Ogata T, Matsuo K, Muro K, Taketo MM, Soga T, Aoki M:Decreased liver B vitamin-related enzymes as a metabolic hallmark of cancer cachexia.Nat Commun, 14(1):6246,2023.10
- Ito N, Tajika M, Tanaka T, Yamada K, Takagi A, Onishi S, Abe T, Higaki E, Fujieda H, Inaba Y, Muro K, Kawashima H, Niwa Y:Skeletal Muscle Quality and Quantity Affect Prognosis after Neoadjuvant Chemotherapy with a Triple Regimen of Docetaxel/Cisplatin/5-FU in Patients with Esophageal Cancer.J Clin Med,12(21):6738,2023.10
- Kajiwara T, Nishina T, Yamashita R, Nakamura Y, Shiozawa M, Yuki S, Taniguchi H, Hara H, Ohta T, Esaki T, Shinozaki E, Takashima A, Yamamoto Y, Yamazaki K, Yoshino T, Hyodo I:Sidedness-Dependent Prognostic Impact of Gene Alterations in Metastatic Colorectal Cancer in the Nationwide Cancer Genome Screening Project in Japan (SCRUM-Japan GI-SCREEN). Cancers (Basel), 15(21):5172,2023.10
- Caughey BA, Umemoto K, Green MF, Ikeda M, Lowe ME, Ueno M, Niedzwiecki D, Taniguchi H, Walden DJ, Komatsu Y, D'Anna R, Esaki T, Denda T, Datto MB, Bando H, Bekaii-Saab T, Yoshino T, Strickler JH, Nakamura Y:Identification of an optimal mutant allele frequency to detect activating KRAS, NRAS, and BRAF mutations in a commercial cell-free DNA next-generation sequencing assay in colorectal and pancreatic adenocarcinomas. J Gastrointest Oncol. 14(5):2083-2096,2023.10
- Ando K, Nakamura Y, Kitao H, Shimokawa M, Kotani D, Bando H, Nishina T, Yamada T, Yuki S, Narita Y, Hara H, Ohta T, Esaki T, Hamamoto Y, Kato K, Yamamoto Y, Minashi K, Ohtsubo K, Izawa N, Kawakami H, Kato T, Satoh T, Okano N, Tsuji A, Yamazaki K, Yoshino T, Maehara Y, Oki E:Mutational spectrum of TP53 gene correlates with nivolumab treatment efficacy in advanced gastric cancer (TP53MUT study). Br J Cancer. 129(6):1032-1039,2023.10
- Shiroyama M, Fukuoka S, Masuishi T, Takashima A, Kumekawa Y, Kajiwara T, Yamazaki K, Shimada Y, Esaki T, Makiyama A, Moriwaki T:Renal impairment as a risk factor for trifluridine/tipiracil-induced adverse events in metastatic colorectal cancer patients from the REGOTAS study. Sci Rep, 13(1):17931,2023.10
- Taniguchi H, Yamazaki K, Masuishi T, Kawakami T, Onozawa Y, Honda K, Kadowaki S, Narita Y, Tsushima T, Hamauchi S, Todaka A, Yokota T, Ando M, Mori K, Shirasu H, Yasui H, Muro K:Bevacizumab, Irinotecan, and Biweekly Trifluridine/Tipiracil for Metastatic Colorectal Cancer: MODURATE, a Phase Ib Study. Oncologist, 2;28(11):e1108-e1113,2023.11
- Suzuki H, Haimoto S, Inaba Y, Tachibana H, Takanari K, Ando M, Yoshizawa K, Hanai N:Peptide Receptor Radionuclide Therapy for Recurrent Olfactory Neuroblastoma After Cranioplasty for Surgical Infection: A Case Report. Anticancer Res, 43(12):5723-5728,2023.12
- Shah MA, Shitara K, Lordick F, Bang YJ, Tebbutt NC, Metges JP, Muro K, Lee KW, Shen L, Tjulandin S, Hays JL, Starling N, Xu RH, Sturtz K, Fontaine M, Oh C, Brooks E, Xu B, Li W, Li CJ, Borodyansky L, Van Cutsem E:Randomized, double-blind, placebo-controlled phase 3 study of paclitaxel {plus minus} napabucasin in pretreated advanced gastric or gastroesophageal junction adenocarcinoma. Clin Cancer Res. 2022 May 26:clincanres, 4021.2021. doi: 10.1158/1078-0432.CCR-21-4021. Online ahead of print.
- Kito Y, Kawakami H, Mitani S, Nishina S, Matsumoto T, Tsuzuki T, Shinohara Y, Shimokawa H, Kumanishi R, Ohta T, Katsuya H, Kawakami T, Nishina T, Hasegawa H, Akiyoshi K, Chiba Y, Yamazaki K, Hironaka S, Muro K:Trifluridine/Tipiracil Plus Bevacizumab for Vulnerable Patients With Pretreated Metastatic Colorectal Cancer: A Retrospective Study (WJOG14520G). Oncologist. 2023 Nov 10:oyad296. doi: 10.1093/oncolo/oyad296. Online ahead of print.
- Shitara K, Yamaguchi K, Muro K, Yasui H, Sakai D, Oshima T, Fujimura M, Sato Y, Yamazaki S, Wakabayashi T, Sugihara M, Kamio T, Shoji H:Trastuzumab deruxtecan in patients with locally advanced or metastatic HER2-positive gastric cancer: a multicenter, open-label, expanded-access study. Int J Clin Oncol. 2023 Nov 14. doi: 10.1007/s10147-023-02422-x. Online ahead of print.
- Narita Y, Matsushima T, Sakamoto Y, Matsuoka H, Tanioka H, Kawakami T, Shoji H, Mizukami T, Izawa N, Nishina T, Yamamoto Y, Mitani S, Nakamura M, Misumi T, Muro K :Chemotherapy after nivolumab for advanced gastric cancer (REVIVE): a prospective observational study. ESMO Open. 2023 Nov 27;8(6):102071. doi: 10.1016/j.esmoop.2023.102071. Online ahead of print.
- Minatogawa H, Izawa N, Shimomura K, Arioka H, Iihara H, Sugawara M, Morita H, Mochizuki A, Nawata S, Mishima K, Tsuboya A, Miyaji T, Honda K, Yokomizo A, Hashimoto N, Yanagihara T, Endo J, Kawaguchi T, Furuya N, Sone Y, Inada Y, Ohno Y, Katada C, Hida N, Akiyama K, Ichikura D, Konomatsu A, Ogura T, Yamaguchi T, Nakajima TE:Dexamethasone-sparing on days 2-4 with combined palonosetron, neurokinin-1 receptor antagonist, and olanzapine in cisplatin: a randomized phase III trial (SPARED Trial). Br J Cancer, 2023 Nov 16. doi: 10.1038/s41416-023-02493-7. Online ahead of print.
和文論文
- 谷口浩也:リモート治験の現状と展望.腫瘍内科 vol.31 No.5,科学評論社:555-560,2023.5
- 松原裕樹、門脇重憲:食道がん診療の進歩と展望(周術期).腫瘍内科 vol.31 No.6,科学評論社:641-647,2023.6
- 谷口浩也:抗がん剤医師主導治験にDCTを実装して感じた課題と展望.腫瘍内科 vol.31 No.6,科学評論社:752-755,2023.6
- 若林宗弘、谷口浩也:微小残存腫瘍の診断と対策.腫瘍内科 vol.32 No.3,科学評論社:309-316,2023.9
- 若林宗弘、室 圭:5章 するっと理解!小腸・大腸のすべて 治療・ケア編 04大腸がん化学療法・薬物療法.解剖生理も、最新の治療も、患者ケアも 決定版!まるごと知りたい消化管,メディカ出版:188-197,2023.10
- 猿田雅之、朴成和、室 圭、沖英次:座談会 がんゲノム医療の現状と今後の課題:2021年の座談会から二年が経過して何がわかったか?.消化器病学サイエンス vol.7 no.3,先端医学社:1-9,2023.9
- 熊西亮介、成田有季哉、室 圭:切除不能進行・再発胃癌の薬物療法.診断と治療 Vol.111 No.7,診断と治療社:〇-〇,2023.7
- 石塚保亘、児玉紘幸、室 圭:Ⅱ各種レジメンの実際One Pint Advice 右側,左側原発部位での分子標的治療薬の使用.Knack&Pitfalls ガイドラインに沿った大腸癌薬物療法の要点と盲点,文光堂:36-37,2023.10
- 児玉紘幸、谷口浩也:Ⅱ各種レジメンの実際 10 MSI-Hや特定の遺伝子異常をもつ大腸癌に対する治療.Knack&Pitfalls ガイドラインに沿った大腸癌薬物療法の要点と盲点,文光堂:84-88,2023.10
- 舛石俊樹、室 圭:10薬物療法の副作用への対応 11.免疫チェックポイント阻害薬による消化器障害について対処法を教えてください.肺癌診療Q&A 一つ上を行く診療の実践第4版,中外医学社:576-578,2023.11
学会発表
- 緒方貴次,舛石俊樹,谷口浩也: 当院における消化器がんゲノム医療推進に向けた完全リモート治験の取り組み. 第109回日本消化器病学会総会,ワークショップ,長崎,2023.4 WS1-6
- 本多和典(演者):Financial Toxicity of Cancer Treatment. 第110回日本泌尿器科学会総会. Panel Discussion,神戸,2023.4月PD2-1
- 室圭(座長):癌治療における支持療法の現状と課題.第48回外科系連合学会学術集会,パネルディスカッション,横浜,2023.6月PD4
- Shoji H, Makiyama A, Katsuya H, Okano N, Negoro Y, Kito Y, Akiyoshi K, Shinozaki K, Yamamoto Y, Kawakami T, Tsuji Y, Kirishima T, Hirao M, Shimura T, Takeshita S, Funakoshi S, Shimokawa M, Yamazaki K, Hironaka S, Muro K: A randomized phase II study comparing S-1 plus oxaliplatin with S-1 monotherapy for elderly patients with advanced gastric cancer: WJOG8315G.IGCC2023, Plenary Session,横浜,2023.6月 PS4
- Wakatsuki T, Ishizuka N, Hironaka S, Minashi K, Kadowaki S, Goto M, Shoji H, Hirano H, Nakayama I, Osumi H, Ogura M, Chin K, Yamaguchi K, Takahari D: Exploratory analysis of HER2 extracellular domain in HER2 positive gastric cancer treated with SOX plus trastuzumab. IGCC2023, Oral Session,横浜,2023.6月 O4-4
- Kodama H, Narita Y, Wakabayashi M, Nakata A, Kumanishi R, Ogata T, Nakazawa T, Matsubara Y, Masuishi T, Honda K, Taniguchi H, Kadowaki S, Ando M, Tajika M, Muro K: Re-administration of nivolumab for advanced gastric cancer patients: a case series. IGCC2023, Poster Session,横浜,2023.6月 P28-2
- Muro K(Chair):Immunotherapy for gastric cancer. IGCC2023, Medical Oncology 4 ,横浜,2023.6月
- Narita Y(Seapker): Upper GI Summit – EGJ Cancer Consensus Conference –Medical oncology, CQ1: Is it recommended to adopt chemotherapy for gastric adenocarcinoma to esophageal adenocarcinoma and esophagogastric junction cancer? IGCC2023, 横浜,2023.6月
- Narita Y(Discussant): Networking sessions for young investigator -sharing optimal treatment strategies. IGCC2023, 横浜,2023.6月
- Muro K(Speaker): How Has Cancer Immunotherapy Changed Gastric Cancer Pharmacotherapy? IGCC2023, Luncheon Seminar ,横浜,2023.6月 LS-1
- Muro K(Speaker): Biomarker-based Treatment Strategies in Advanced Gastric and Gastroesophageal Junction Cancer. IGCC2023, Morning Seminar ,横浜,2023.6月 MS2-1
- Taniguchi H, Yagisawa M, Satoh T, Kadowaki S, Sunakawa S, Nishina T, Komatsu Y, Esaki T, Sakai D, Doi A, Kajiwara T, Ono H, Asano M, Hirano N, Odegaard JI, Fujii S, Nomura S, Sato A, Yoshino T, Nakamura Y: Tissue-agnostic efficacy of trastuzumab deruxtecan (T-DXd) in advanced solid tumors with HER2 amplification identified by plasma cell-free DNA (cfDNA) testing: Results from a phase 2 basket trial (HERALD/EPOC1806). ASCO 2023, Poster Discussion Session, Chicago,2023.6月 3014
- Jogo T, Shinozaki E, Masuishi T, Kato T, Nishina T, Esaki T, Komatsu Y, Kato K, Suzuki M, Fuse N, Sato A, Ikeno T, Nomura S, Bando H, Odegaard JI, Fujii S, Ebi H, Yoshino T, Nakamura Y: Efficacy and safety of futibatinib for refractory advanced solid malignancies with FGFR alterations identified in circulating tumor DNA: TiFFANY, A GOZILA-affiliated Trial. ASCO 2023, Poster Session, Chicago,2023.6月 3102
- Matsubara J, Mukai K, Yoshioka M, Kage H, Oda K, Kudo R, Ikeda S, Ebi H, Muro K, Hayashi R, Tokudome N, Yamamoto N, Muto M: Comprehensive genomic profiling before the first-line setting versus after the completion of standard of care in patients with previously untreated advanced solid tumors: The prospective FIRST-Dx study.ASCO 2023, Poster Session, Chicago,2023.6月 3111
- Osumi H, Shinozaki E, Nakamura Y, Esaki T, Yasui H, Taniguchi H, Satake H, Sunakawa Y, Komatsu Y, Kagawa Y, Denda T, Shiozawa M, Satoh T, Nishina T, Goto M, Takahashi N, Kato T, Bando H, Yamaguchi K, Yoshino T: NeoRAS wild-type metastatic colorectal cancer in the SCRUM-Japan GOZILA study. ASCO 2023, Oral Abstract Session, Chicago,2023.6月 3506
- Yamazaki K, Muro K, Watanabe J, Shitara K, Ohori H, Shiozawa M, Yasui H, Oki E, Sato T, Naito T, Komatsu Y, Kato T, Soeda J, Yamamoto K, Yamashita R, Akagi K, Ochiai A, Uetake H, Tsuchihara K, Yoshino T: Efficacy of panitumumab in patients with left-sided disease, MSS/MSI-L, and RAS/BRAF WT: A biomarker study of the phase III PARADIGM trial.ASCO 2023, Oral Abstract Session, Chicago,2023.6月 3508
- Hong DS, Kuboki Y, Strickler JH, Fakih M, Houssiau Hln, Price TJ, Elez E, Siena S, Chan E, Nolte-Hippenmeyer J, Cardona P, Tran Q, Masuishi T: Sotorasib (Soto) plus panitumumab (Pmab) and FOLFIRI for previously treated KRAS G12Cmutated metastatic colorectal cancer (mCRC): CodeBreaK 101 phase 1b safety and efficacy. ASCO 2023, Poster Discussion Session, Chicago,2023.6月 3513
- Oki E, Kotani D, Nakamura Y, Mishima S, Bando H, Yukami H, Ando K, Miyo M, Watanabe J, Hirata K, Akazawa N, Yeh K-H, Laliotis G, Sharma S, Liu M, Taniguchi H, Takemasa I, Kato T, Mori M, Yoshino T: Circulating tumor DNA dynamics as an early predictor of recurrence in patients with radically resected colorectal cancer: Updated results from GALAXY study in the CIRCULATE-Japan. ASCO 2023, Poster Discussion Session, Chicago,2023.6月 3521
- Bando H, Satake H, Kotani D, Hamaguchi T, Shiozawa M, Ikumoto T, Okita Y, Masuishi T, Kagawa Y, Yasui H, Oki E, Komatsu Y, Taniguchi H, Muro K, Kotaka M, Yamazaki K, Misumi T, Yoshino T, Kato T, Tsuji A: A multicenter randomized phase II trial comparing CAPOXIRI + bevacizumab with FOLFOXIRI+ bevacizumab as first-line treatment in patients with metastatic colorectal cancer:Primary results of the QUATTRO-II study.ASCO 2023, Poster Session, Chicago,2023.6月 3565
- Kuwata T, Nakamura Y, Nishina T, Takahashi N, Narimatsu H, Cho H, Inagaki C, Kadowaki S, Okugawa Y, Matsuzaki S, Tomita N, Yasui H, Sunakawa Y, Matsukawa M, Kimura K, Hiraoka Y, Hirata M, Imoto I, Kosugi S, Yoshino T: Utility of multi-gene panel-based germline analysis following genomic profiling and cascade testing in advanced solid tumors: An initial report of the BRANCH study. ASCO 2023, Poster Session, Chicago,2023.6月 10615
- 門脇重憲(演者):甲状腺がんにおける分子標的治療の現状と展望. 第47回日本頭頸部癌学会総会・学術講演会,シンポジウム(Precision Medicine 新世紀).大阪,2023.6月 S5-2
- 門脇重憲,岡 弘毅,長島健悟,西 秀昭,熊井良彦,飯島宏章,大上研二,清水 康,加納里志,伊東和恵,山崎知子,高橋秀聡,折舘伸彦,横田知哉,小山泰司,清田尚臣,佐藤靖祥,高橋俊二,加藤恭子,本間義崇: 白金製剤・ニボルマブ不応・不耐後の再発・転移頭頸部扁平上皮癌の臨床転帰に関する後方視的多施設共同研究.第47回日本頭頸部癌学会総会・学術講演会,口演,大阪,2023.6月 O-043
- 室圭(座長): 2023年6月、がん悪液質治療の現在地「アナモレリン塩酸塩の最適な使用タイミングを考える」. 第8回日本がんサポーティブケア学会学術集会,イブニングセミナー,奈良,2023.6月
- 緒方貴次(演者): 2023年6月、がん悪液質治療の現在地「アナモレリン塩酸塩の最適な使用タイミングを考える」. 第8回日本がんサポーティブケア学会学術集会,イブニングセミナー,奈良,2023.6月
- 門脇重憲(司会):CDDP unfit食道癌に対する治療戦略. 第77回 日本食道学会学術集会,要望演題,大阪,2023.6月
- 宮田博志、牧野知紀、安田卓司、北川雄光、室圭、朴 在賢、引地哲郎、長谷川 貴大、五十嵐憲二、塩崎均: 食道癌患者に対するS-588410 術後補助療法の第3相プラセボ対照無作為化試験. 第77回 日本食道学会学術集会,プレナリーセッション,大阪,2023.6月PS1-1
- 児玉紘幸、門脇重憲、田近正洋、桧垣栄治、藤枝裕倫、安部哲也、尾瀬功、室圭: 切除不能局所進行食道癌に対する術前DCF 療法におけるR0 切除予測因子の検討. 第77回 日本食道学会学術集会, シンポジウム ,大阪,2023.6月SY3-1
- 加藤 健、小島隆嗣、原 浩樹、辻 晃仁、安井久晃、室圭、佐藤太郎、八束尚良、韓 士栄、土井俊彦: KEYNOTE-590 results in Japanese patients after an additional 1-year of follow-up. 第77回 日本食道学会学術集会, パネルディスカッション,大阪,2023.6月PD3-3
- 白石和寛、杉山圭司、下嵜啓太郎、岡田真央、松原裕樹、廣瀬 優、土橋賢司、舛石俊樹、平田賢郎: 切除不能食道扁平上皮癌におけるFOLFOX 療法に関する多施設後ろ向き観察研究. 第77回 日本食道学会学術集会,要望演題,大阪,2023.6月R9-4
- 山本 駿、陳 勁松、小島隆嗣、西野和美、川上尚人、小柳和夫、田中善宏、飯島 克則、室圭: 固形癌及び食道癌に対するFutibatinib/ ペムブロリズマブ併用療法の臨床第1b 相試験. 第77回 日本食道学会学術集会, ポスター ,大阪,2023.6月P28-11
- Kato T,Kotani D,Takashima D,Satoh T, Masuishi T, Komatsu Y, Shiozawa M, Esaki T, Izawa N,Takeuchi S, Bando H, Iwasa S,Hasegawa H,Yamaguchi T, Taniguchi H, Yamada S, Yoshino T: Clinical outcomes of encorafenib, binimetinib, and cetuximab for pretreated BRAF V600E-mutant metastatic colorectal cancer in the BEACON EAP follow-up study.ESMO GI 2023,Poster, Barcelona,2023.6月 P-23
- Yamada T,Kito Y,Sakai K,Nishio K,Yamazaki K, Shoji H,TsushimaT,Mitani S, Shiraishi K, Yasui H,Hara H,Shimozaki K, Esaki T, Shimokawa H,Tsuzuki T, Kajiura S, Masuishi T, Baba E, Yoshimura K, Kawakami H, Hironaka S, Muro K: Analysis of plasma angiogenesis factors on the efficacy of FOLFIRI plus ramucirumab and FOLFOXIRI plus ramucirumab as first-line treatment for metastatic colorectal cancer from WJOG9216G randomized phase II study. ESMO GI 2023,Poster, Barcelona,2023.6月 P-80
- Kotaka M, Bando H, Satake H, Kotani D,Hamaguchi T, Shiozawa M, Ikumoto T, Masuishi T, Yasui H, Kagawa Y, Oki E, Yamamoto Y, Kawakami H, Boku S, Komatsu Y, Taniguchi H, Muro K, Yamazaki K, Misumi T, Yoshino T, Kato T, Tsuji A: QUATTRO-II: A multicenter randomized trial comparing CAPOXIRI + bevacizumab with FOLFOXIRI + bevacizumab as first-line treatment in patients with metastatic colorectal cancer: Efficacy and safety analysis. ESMO GI 2023,Poster, Barcelona,2023.6月 P-149
- Ogata T, Harada K, Kawakami T, Hu Q, Kadowaki S, Taniguchi H, Muro K, Yamamura T,Kawamoto Y, Komatsu Y, Fushiki K, Oshima K, Nakanishi R, Ando K, Nambara S, Masuishi T, Yamazaki K, Oki E, Yuki S: Comparison of treatment outcomes of regorafenib for patients with metastatic colorectal cancer by era: A propensity-score matched analysis. ESMO GI 2023,Poster, Barcelona,2023.6月 P-199
- 舛石俊樹(座長):免疫チェックポイント阻害薬治療を受けているがん患者の緩和ケアに携わる医療者が知っておきたい事. 第28回日本緩和医療学会学術大会,ランチョンセミナー,神戸,2023.7月 LS16
- 室圭(座長):大腸がん診療におけるリキッドバイオプシーの可能性. 第99回大腸癌研究会学術集会,ランチョンセミナー,尼ケ崎,2023.7月LSF
- 谷口浩也(演者):移りゆく大腸癌個別化治療のこれから. 第99回大腸癌研究会学術集会,ランチョンセミナー,尼ケ崎,2023.7月LSE
- 室圭(演者):EvidenceとNarrativeの融合 ~BRAF変異陽性大腸癌の個別化治療とがん悪液質治療~. Best of ASCO 2023 in Japan, オンコロジーライブセミナー,WEB開催,2023.7月
- 門脇重憲(司会):消化器がん- Gastrointestinal. Best of ASCO 2023 in Japan,口演 ,WEB開催,2023.7月
- 室圭(演者):高頻度マイクロサテライト不安定性を有する胆道癌でのペムブロリズマブ使用成績調査―適正使用委員会からの報告―. 第59回日本胆道学会学術集会,シンポジウム,札幌,2023.9月 S2-5
- 谷口浩也(座長):Breakthrough approaches deciphering carcinogenesis or progression of CRC. 第82回日本癌学会学術総会, English Oral Sessions,横浜,2023.9月 E14-2
- 谷口浩也(演者):Decentralized clinical trials - current status and future perspectives -.第82回日本癌学会学術総会, Special Symposia,横浜,2023.9月 SS7-3
- 本多和典(演者):進行性腎癌における薬物治療に対する治療選好と経済毒性「がんの経済毒性」.第88回日本泌尿器科学会 東部総会,イブニングセミナー,札幌,2023.10月 ES2-2
- 安藤正志(演者):後腹膜肉腫に対する内科治療. 第61回日本癌治療学会学術集会, 領域横断シンポジウム,横浜,2023.10月 CCSP8-5
- 高山浩一、小島 愛、本田主税、木口舞美、遠藤俊充、室圭: アナモレリンの副作用および治療効果の予測因子:特定使用成績調査の中間解析, 第61回日本癌治療学会学術集会, “Frontier” ,横浜,2023.10月 FR4-4
- 松本繁巳、武藤 学、福山啓太、山本秀和、室圭、砂川 優、安井久晃、大槻 涼、木村裕一、岡田昌史、西浦亮二、湯川洋一郎:がん診療におけるリアルワールドデータ(RWD)収集に関する多施設共同研究. 第61回日本癌治療学会学術集会,eポスター,横浜,2023.10月 P47-5
- 成田有季哉(演者): 抗HER2 療法の治療変革. 第61回日本癌治療学会学術集会, スポンサードシンポジウム, 横浜,2023.10月 SPSY1-4
- 篠崎英司、城後友望子、衣斐寛倫、舛石俊樹、加藤健志、仁科智裕、江﨑泰斗、小松嘉人、加藤 健、佐藤暁洋、坂東英明、J.Odegaard、藤井誠志、吉野孝之、中村能章:ctDNAにFGFR遺伝子異常を有する進行固形がんに対するフチバチニブの有効性の検討. 第61回日本癌治療学会学術集会, “Frontier” ,横浜,2023.10月 FR4-2
- 胡 慶江、原田一顕、川上武志、緒方貴次、伏木邦博、谷口浩也、室圭、安藤幸滋、南原 翔、川本泰之、小松嘉人、沖 英次、舛石俊樹、山﨑健太郎、結城敏志:転移性大腸癌におけるレゴラフェニブの開始用量が治療成績に対する影響. 第61回日本癌治療学会学術集会, 口演,横浜,2023.10月 O15-5
- 舛石俊樹(演者):切除不能進行・再発胃癌に対する薬物療法の開発. 第61回日本癌治療学会学術集会,臓器別ワークショップ,横浜,2023.10月 OWS9-1
- 安井久晃、坂東英明、佐竹悠良、小谷大輔、濱口哲弥、小髙雅人、谷口浩也、室圭、小松嘉人、沖 英次、山崎健太郎、三角俊裕、吉野孝之、加藤健志、辻 晃仁. 進行大腸癌に対するCAPOXIRI+Bev 療法とFOLFOXIRI+Bev 療法のランダム化第II 相臨床研究. 第61回日本癌治療学会学術集会, アンコールセッション,横浜,2023.10月 EN1-3
- 安藤幸滋、沖 英次、小谷大輔、中村能章、三島沙織、三代雅明、浜部敦史、渡邉 純、小髙雅人、Aushev.V、谷口浩也、山崎健太郎、竹政伊知朗、加藤健志、吉野孝之:MRD は手術先行直腸癌患者の再発予測因子である:GALAXY 試験のアップデート結果より. 第61回日本癌治療学会学術集会, “Frontier” ,横浜,2023.10月 FR1-4
- 濱口哲弥、小谷大輔、塩澤 学、坂東英明、佐竹悠良、沖 英次、小松嘉人、谷口浩也、室圭、小髙雅人、山﨑健太郎、三角俊裕、吉野孝之、加藤健志、辻 晃仁: UGT1A1 遺伝子型がQUATTRO-II 試験の有効性と安全性に及ぼす影響. 第61回日本癌治療学会学術集会, “Frontier” ,横浜,2023.10月 FR1-6
- 谷口浩也(演者):秋澄む横浜で伝えたい大腸癌薬物療法の急所. 第61回日本癌治療学会学術集会,学術セミナー ,横浜,2023.10月 LS4
- 谷田部 恭、織田克利、谷口浩也、角南久仁子、安岡正太郎、渡部亮介:本邦における臓器横断的CDXとCGP検査のリアルワールドデータ. 第61回日本癌治療学会学術集会,口演 ,横浜,2023.10月 O3-4
- 谷口浩也、Büntzel J、矢野琢也、木村純子、中村将人、Velthuis E、 Jawad Hamad J、Garcia-Foncillas J:実地診療でのセツキシマブ使用経験:インフュージョンリアクション、皮膚毒性を中心に.第61回日本癌治療学会学術集会,口演 ,横浜,2023.10月 O15-3
- 谷口浩也(演者):遠隔転移巣切除後補助化学療法におけるshared decision makingの実践. 第61回日本癌治療学会学術集会,臓器別シンポジウム ,横浜,2023.10月 OSP8-1
- 本多和典(演者):がんの経済毒性~現状・課題・今後~. 第61回日本癌治療学会学術集会,学術セミナー ,横浜,2023.10月 LS50
- 橋本直佳、藤澤孝夫、加藤大悟、森實千種、岩田広治、岡野 晋、山上 亘、山崎直也、門脇重憲、上野 誠、尾崎洋史、飯田直子、中村能章、坂東英明、吉野孝之:SCRUM-Japan 基盤に紐づくバイオマーカー適合治験における有効性の検討.第61回日本癌治療学会学術集会, “Frontier” ,横浜,2023.10月 FR4-3
- 對馬隆浩、松田 諭、川久保博文、山本 駿、竹内裕也、細川 歩、門脇重憲、渡辺晃識、吉井貴子、尾形高士、陳 勁松、小谷大輔、平田賢郎、浜本康夫、北川雄光:切除可能食道扁平上皮癌に対する術前FLOT 療法の第II 相試験―初期安全性評価の報告―.第61回日本癌治療学会学術集会,口演 ,横浜,2023.10月 O2-3
- 酒井 真、佐伯浩司、加藤 健、門脇重憲、笠原佑記、佐藤雄亮、三梨桂子、山本 駿、小柳和夫、伊澤直樹、野村基雄、安田知代、伊澤真木子、上村鋼平、室圭: 進行・再発食道がん患者の治療体系と予後に関する観察研究(POME). 第61回日本癌治療学会学術集会,口演 ,横浜,2023.10月 O5-4
- 松本繁巳、門脇重憲、町田望、仁科智裕、砂川 優、前北隆雄、西岡真理子、後藤知之、浜本康夫、安井久晃、川邉里紗、岡田昌史、武藤 学、室圭: Cyber Oncology® を活用した進行胃癌治療のreal-world data 構築のための実行可能性研究. 第61回日本癌治療学会学術集会,口演 ,横浜,2023.10月 O28-4
- 三梨桂子、川上尚人、仁科慎一、門脇重憲、松岡宏、大塚倫之、藪崎裕、馬場秀夫、保坂尚志、松原淳一、仁科智裕、君嶋悠矢、松田祐子、武藤学、室圭: 胃癌患者に対するニボルマブ+ 化学療法の有効性と安全性に関する観察研究(中間報告). 第61回日本癌治療学会学術集会,口演 ,横浜,2023.10月 O78-4
- Nakamura Y, Kotani D, Saori M, Yukami H, Watanabe J, Akazawa N, Kataoka K, Hirata K, Yamazaki K, Yeh K, Laliotis G, Aushev V, Jurdi A, Kotaka M, Bando H, Taniguchi H, Takemasa I, Kato T, Yoshino T, Oki E: Circulating tumor (ct)DNA as a prognostic biomarkerin patients (pts) with resected colorectal cancer (CRC): Anupdated 24 months (mos) disease free survival (DFS) analysisfrom GALAXY study (CIRCULATE-Japan). ESMO Congress 2023, Mini oral session, Madrid,2023.10月 558MO
- Yuki S, Harada K, Kawakami T, Ogata T, Hu Q, FUSHIKI K, Oshima K, Kadowaki S, Taniguchi H, Muro K, Nakanishi R, Ando K, Nambara S, Nakamura T, Kawamoto Y, Komatsu Y, Oki E, Masuishi T, Yamazaki K: The impacts of starting regorafenib dose on treatmentoutcomes in metastatic colorectal cancer. ESMO Congress 2023, Poster session , Madrid,2023.10月 615P
- Muro K, Kawakami H, Kadowaki S, Makiyama A, Tsuda M, Hirata K, Sugimoto N, Machida N, Hara H, Hirano H, Esaki T, Komatsu Y, Hironaka S: A phase II study of nivolumab plus low doseipilimumab as fi rst -line therapy in patients with advancedgastric or esophago-gastric junction MSI-H tumor: First resultsof the NO LIMIT study (WJOG13320G/CA209-7W7). ESMO Congress 2023, Mini oral session, Madrid,2023.10月 1513MO
- Yamamoto S, Muro K, Nishino K, Kawakami H, Kojima T, Ojima H, Tanaka Y, Suyama K, Machida N, Koyanagi K, Iijima K, Hirai H, Chisamore M, Chin K: Phase Ib study of futibatinib plus pembrolizumab inpatients with esophageal carcinoma: Updated results ofantitumor activity and tolerability results in combination withchemotherapy. ESMO Congress 2023, Poster session , Madrid,2023.10月 1532P
- Hirano H, Kawazoe A, Yamaguchi K, Hamaguchi T, Narita Y, Boku S, Oshima T, Hara H, Hamamoto Y, Esaki T, Ishido K: Safety, effi cacy, and biomarkers for ONO-4578 plus nivolumab in unresectable advanced or recurrent gastric or gastroesophageal cancer. ESMO Congress 2023, Poster session, Madrid, 2023.10月 1546P
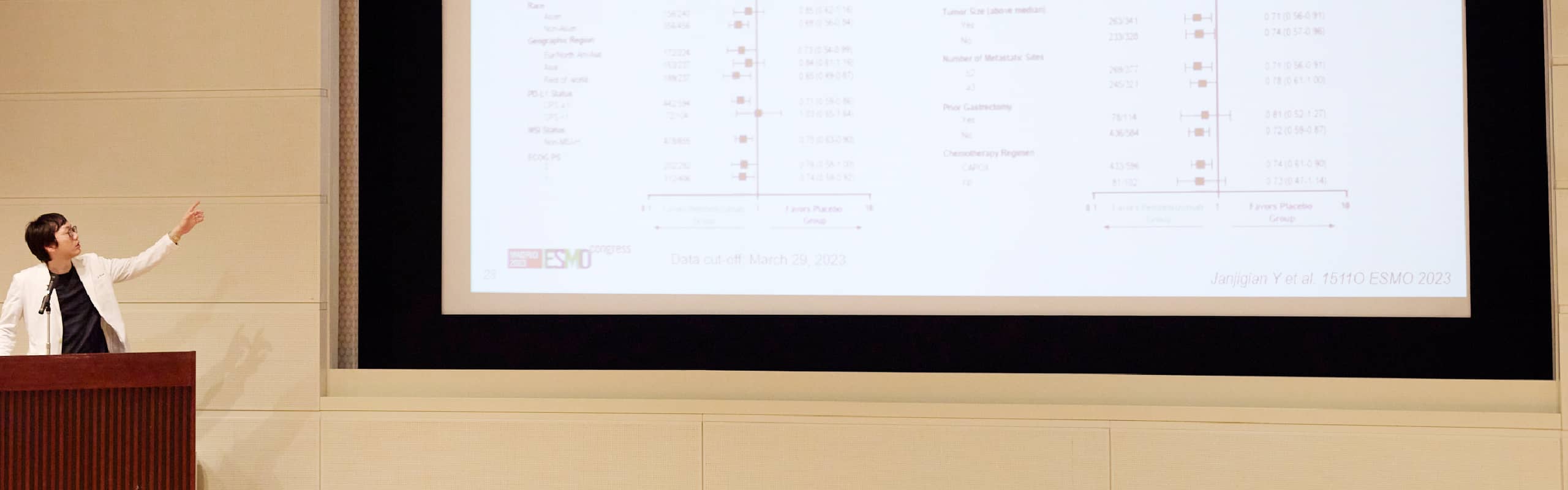
- Janjigian YY, Al-Batran S, Wainberg ZA, Van Cutsem E, Molena D, Muro K, Hyung WJ, Wyrwicz LS, Oh D, Omori T, Moehler M, Garrido M, Cunha Sousa Oliveira S, Liberman M, Castro Oliden V, Bilici M, Kurland JF, Xynos I, Mann H, Tabernero J: Pathological complete response (pCR) to durvalumab plus 5-fl uorouracil, leucovorin, oxaliplatin and docetaxel (FLOT) in resectable gastric and gastroesophageal junction cancer (GC/GEJC): Interim results of the global, phase III MATTERHORN study. ESMO Congress 2023, Proffered Paper session, Madrid, 2023.10月 LBA73
- Kadowaki S, Sakai D, Kawabata R, Hara H, Yasui H, Takahashi M, Hirao M, Imai H, Minashi K, Kawakami T, Satake H, Matsuyama J, Sakamoto Y, Sawada K, Kataoka M, Kawakami H, Shimokawa T, Boku N, Satoh T: The primary results of an intergroup phase III randomized controlled trial comparing ramucirumab plus irinotecan with irinotecan in the third or later line treatment beyond progression after ramucirumab for advanced gastric cancer (RINDBeRG trial). ESMO Congress 2023, Proffered Paper session, Madrid, 2023.10月 LBA76
- 本多和典(演者):なぜいま就労両立支援が重要なのか?~第4期がん対策推進基本計画とアフターコロナを踏まえて~「がんの経済毒性」. 第64回日本肺癌学会学術集会, 教育研修委員会企画就労両立支援セッション, 千葉, 2023.11月 就労-2
- 舛石俊樹、小森康司、室圭:切除不能再発直腸癌における局所再発が予後や化学療法の有効性に与える影響. 日本消化器関連学会(JDDW 2023), シンポジウム, 神戸, 2023.11月 外S11-8指
- 谷口浩也(演者): MSI-H大腸癌をもっと知ろう!学ぼう!. 日本消化器関連学会(JDDW 2023), サテライトシンポジウム, 神戸, 2023.11月 サテ75-1
- 谷口浩也(演者): 遠隔治験の経験から考えるがん診療における遠隔医療. 第27回日本遠隔医療学会学術大会, 腫瘍内科遠隔医療分科会, 新潟, 2023.11月 SC3-1
- Kato K, Muro K, Watanabe J, Shitara K, Yamazaki K, Ohori H, Shiozawa M, Yasui H, Oki E, Sato T, Naitoh T, Komatsu Y, Soeda J, Yamamoto K, Yamashita R, Akagi K, Ochiai A, Uetake H, Tsuchihara K, Yoshino T: Panitumumab (PAN) vs bevacizumab (BEV) in metastatic colorectal cancer (mCRC) by microsatellite stable (MSS), RAS/BRAF, and HER2 amplification (HER2amp) status: Phase III PARADIGM biomarker study. ESMO Asia Congress 2023, Mini Oral session, Singapore, 2023.12月 90MO
- Masuishi T, Strickler J, Machiels J.P, Hong D, Greil R, Chan E, Hippenmeyer J, Saportas Y, Cardona P, Xia C, Kuboki Y: Sotorasib (Soto) + panitumumab (Pmab) and FOLFIRI in previously treated KRASG12C-mutated metastatic colorectal cancer (mCRC): Safety and efficacy for CodeBreaK 101 phase Ib expansion cohort. ESMO Asia Congress 2023, Mini Oral session, Singapore, 2023.12月 92MO
- Kotani D, Yoshino T, Paulson A, Masuishi T, Hochster H, Krauss J, Sunakawa Y, Takashima A, Yamazaki K, Kawakami H, Nishina T, Komatsu Y, Esaki T, Eng C, Dasari N.A, Yu Z, Chen L, Yang T, Schelm W: Efficacy and safety of fruquintinib (F) + best supportive care (BSC) vs placebo (P) + BSC in refractory metastatic colorectal cancer (mCRC): Asian vs non-Asian outcomes in FRESCO-2. ESMO Asia Congress 2023, Poster session, Singapore, 2023.12月 93P
- Kajiwara T, Nishina T, Yamashita R, Nakamura Y, Shiozawa M, Yuki S, Taniguchi H, Hara H, Ohta T, Esaki T, Shinozaki E, Takashima A, Yamamoto Y, Yamazaki K, Yoshino T, Hyodo I: Sidedness-dependent prognostic impact of gene alterations in metastatic colorectal cancer in the nationwide cancer genome screening project in Japan (SCRUM-Japan GI-SCREEN). ESMO Asia Congress 2023, Poster session, Singapore, 2023.12月 94P
- Yoshino T, Hooda N, Younan D, Muro K, Shitara K, Heinemann V, O'Neil B, Herrero F.R, Peeters M, Soeda J, Suh M, Reichart H, Mezzi K, Fryzek J, Chia V, Rehn M, Stintzing S: A meta-analysis of efficacy and safety from head-to-head first-line (1L) trials of epidermal growth factor receptor inhibitors (EGFRIs) versus bevacizumab in combination with chemotherapy (CT) doublets in patients with RAS wild-type (WT) metastatic colorectal cancer (mCRC) by sidedness. ESMO Asia Congress 2023, Poster session, Singapore, 2023.12月 127P
- Oh DY, Janjigian Y, Al-Batran SE, Wainberg Z, Cutsem EV, Molena D, Muro K, Hyung WJ, Wyrwicz L, Omori T, Moehler M, Garrido M, Oliveira SCS, Liberman M, Oliden VC, Bilici M, Kurland J, Xynos I, Mann H, Tabernero J: Pathological complete response (pCR) to durvalumab plus 5-fluorouracil, leucovorin, oxaliplatin and docetaxel (FLOT) in resectable gastric and gastroesophageal junction cancer (GC/GEJC): Interim results of the global, phase III MATTERHORN study. ESMO Asia Congress 2023, Oral session, Singapore, 2023.12月 129O
- Narita Y, Komori A, Hironaka S, Kawakami H, Furuta M, Kawakami T, Makiyama A, Sugiyama K, Takegawa N, Hirano H, Ando T, Matsushima T, Chida A, Kashiwada T, Komoda M, Matsumoto T, Oda H, Yabusaki H, Yoshimura K, Muro K: A prospective observational study of MSI screening in unresectable chemotherapy-naïve advanced gastric cancer/gastroesophageal junction cancer: WJOG13320GPS. ESMO Asia Congress 2023, Poster session, Singapore, 2023.12月 149P
英文論文
- Kajimoto Y, Shibutani T, Nagao S, Yamaguchi S, Suzuki S, Mori M, Tsubouchi H, Nakao K, Azuma A, Koyanagi T, Kohara I, Tamaki S, Yabuki M, Teng L, Honda K, Igarashi A:Validity of the COmprehensive Score for financial Toxicity (COST) in patients with gynecologic cancer. Int J Gynecol Cancer, ijgc-2022-003410,2022.4
- Miyo M, Kato T, Nakamura Y, Taniguchi H, Takahashi Y, Ishii M, Okita K, Ando K, Yukami H, Mishima S, Yamazaki K, Kotaka M, Watanabe J, Oba K, Aleshin A, Billings PR, Rabinowitz M, Kotani D, Oki E, Takemasa I, Mori M, Yoshino T:DENEB: Development of new criteria for curability after local excision of pathological T1 colorectal cancer using liquid biopsy. Cancer Sci, 113(4):1531-1534,2022.4
- Miyo M, Kato T, Nakamura Y, Taniguchi H, Takahashi Y, Ishii M, Okita K, Ando K, Yukami H, Mishima S, Yamazaki K, Kotaka M, Watanabe J, Oba K, Aleshin A, Billings PR, Rabinowitz M, Kotani D, Oki E, Takemasa I, Mori M, Yoshino T:DENEB: Development of new criteria for curability after local excision of pathological T1 colorectal cancer using liquid biopsy. Cancer Sci, 113(4):1531-1534,2022.4
- Bando H, Nakamura Y, Taniguchi H, Shiozawa M, Yasui H, Esaki T, Kagawa Y, Denda T, Satoh T, Yamazaki K, Sunakawa Y, Kato T, Goto M, Yuki S, Nishina T, Oki E, Shinozaki E, Matsuhashi N, Takahashi N, Tsuji A, Ohtsubo K, Wakabayashi M, Ikeno T, Hata M, Odegaard JI, Yoshino T:Effects of Metastatic Sites on Circulating Tumor DNA in Patients With Metastatic Colorectal Cancer. JCO Precis Oncol, 6:e2100535,2022.4
- Naito T, Uchino J, Kojima T, Matano Y, Minato K, Tanaka K, Mizukami T, Atagi S, Higashiguchi T, Muro K, Takayama K, Furuse J, Morishima E, Takiguchi T, Tamura K:A multicenter, open-label, single-arm study of anamorelin (ONO-7643) in patients with cancer cachexia and low body mass index. Cancer, 128(10):2025-2035,2022.5
- Hasegawa H, Shitara K, Takiguchi S, Takiguchi N, Ito S, Kochi M, Horinouchi H, Kinoshita T, Yoshikawa T, Muro K, Nishikawa H, Suna H, Kodera Y:A multicenter, open-label, single-arm phase I trial of neoadjuvant nivolumab monotherapy for resectable gastric cancer. Gastric Cancer, 25(3):619-628,2022.5
- Kikuchi K, Yamazaki N, Nozawa K, Fukuda H, Shibata T, Machida R, Hamaguchi T, Takashima A, Shoji H, Boku N, Takatsuka S, Takenouchi T, Nishina T, Yoshikawa S, Takahashi M, Hasegawa A, Kawazoe A, Masuishi T, Mizutani H, Kiyohara Y:Topical corticosteroid therapy for facial acneiform eruption due to EGFR inhibitors in metastatic colorectal cancer patients: a randomized controlled trial comparing starting with a very strong or a weak topical corticosteroid (FAEISS study, NCCH1512, colorectal part). Support Care Cancer, 30(5):4497-4504,2022.5
- Matsumura M, Hasegawa K, Oba M, Yamaguchi K, Uetake H, Yoshino T, Morita S, Takahashi K, Unno M, Shimada Y, Muro K, Matsuhashi N, Mori M, Baba H, Shimada M, Mise Y, Kawaguchi Y, Kagimura T, Ishigure K, Saiura A, Sugihara K, Kokudo N:A randomized controlled trial of surgery and postoperative modified FOLFOX6 versus surgery and perioperative modified FOLFOX6 plus cetuximab in patients with KRAS wild-type resectable colorectal liver metastases: EXPERT study. Langenbecks Arch Surg, 407(4):1345-1356,2022.6
- Akazawa N, Itoh N, Ando M:A man with fever and aortitis. Eur J Intern Med, 100:123-124,2022.6
- Kawakami T, Masuishi T, Kawamoto Y, Go H, Kato K, Kumanishi R, Sawada K, Yuki S, Yamamoto K, Komatsu Y, Muro K, Fushiki K, Shirasu H, Yamazaki K:The survival benefit of increasing the number of active drugs for metastatic colorectal cancer: A multicenter retrospective study. Cancer Med, 11(11):2184-2192,2022.6
- Janjigian YY, Van Cutsem E, Muro K, Wainberg Z, Al-Batran SE, Hyung WJ, Molena D, Marcovitz M, Ruscica D, Robbins SH, Negro A, Tabernero J:MATTERHORN: phase III study of durvalumab plus FLOT chemotherapy in resectable gastric/gastroesophageal junction cancer. Future Oncol, 18(20):2465-2473,2022.6
- Ooki A, Satoh T, Muro K, Takashima A, Kadowaki S, Sakai D, Ichimura T, Mitani S, Kudo T, Chin K, Kitano S, Thai D, Zavodovskaya M, Liu J, Boku N, Yamaguchi K:A phase 1b study of andecaliximab in combination with S-1 plus platinum in Japanese patients with gastric adenocarcinoma. Sci Rep, 12(1):11007,2022.6
- Matsumura M, Hasegawa K, Oba M, Yamaguchi K, Uetake H, Yoshino T, Morita S, Takahashi K, Unno M, Shimada Y, Muro K, Matsuhashi N, Mori M, Baba H, Shimada M, Mise Y, Kawaguchi Y, Kagimura T, Ishigure K, Saiura A, Sugihara K, Kokudo N:A randomized controlled trial of surgery and postoperative modified FOLFOX6 versus surgery and perioperative modified FOLFOX6 plus cetuximab in patients with KRAS wild-type resectable colorectal liver metastases: EXPERT study. Langenbecks Arch Surg, 407(4):1345-1356,2022.6
- Fujii S, Kotani D, Hattori M, Nishihara M, Shikanai T, Hashimoto J, Hama Y, Nishino T, Suzuki M, Yoshidumi A, Ueno M, Komatsu Y, Masuishi T, Hara H, Esaki T, Nakamura Y, Bando H, Yamada T, Yoshino T:Rapid Screening Using Pathomorphologic Interpretation to Detect BRAFV600E Mutation and Microsatellite Instability in Colorectal Cancer. Clin Cancer Res, 28(12):2623-2632,2022.6
- Itoh N, Akazawa N, Ishibana Y, Masuishi T, Nakata A, Murakami H:Clinical and microbiological features of obstructive cholangitis with bloodstream infection caused by Pandoraea apista identified by MALDI-TOF mass spectrometry and ribosomal RNA sequencing in a cancer patient. BMC Infect Dis, 22(1):529,2022.6
- Sundar R, Huang KK, Kumar V, Ramnarayanan K, Demircioglu D, Her Z, Ong X, Bin Adam Isa ZF, Xing M, Tan AL, Tai DWM, Choo SP, Zhai W, Lim JQ, Das Thakur M, Molinero L, Cha E, Fasso M, Niger M, Pietrantonio F, Lee J, Jeyasekharan AD, Qamra A, Patnala R, Fabritius A, De Simone M, Yeong J, Ng CCY, Rha SY, Narita Y, Muro K, Guo YA, Skanderup AJ, So JBY, Yong WP, Chen Q, Göke J, Tan P:Epigenetic promoter alterations in GI tumour immune-editing and resistance to immune checkpoint inhibition. Gut, 71(7):1277-1288,2022.7
- Shah MA, Shitara K, Lordick F, Bang YJ, Tebbutt NC, Metges JP, Muro K, Lee KW, Shen L, Tjulandin S, Hays JL, Starling N, Xu RH, Sturtz K, Fontaine M, Oh C, Brooks EM, Xu B, Li W, Li CJ, Borodyansky L, Van Cutsem E:Randomized, Double-Blind, Placebo-Controlled Phase III Study of Paclitaxel ± Napabucasin in Pretreated Advanced Gastric or Gastroesophageal Junction Adenocarcinoma. Clin Cancer Res, 14:OF1-OF9,2022.7
- Mitani S, Kato K, Daiko H, Ito Y, Nozaki I, Kojima T, Yano M, Nakagawa S, Ueno M, Watanabe M, Tsunoda S, Abe T, Kadowaki S, Kadota T, Sasaki K, Machida R, Kitagawa Y:Second primary malignancies in patients with clinical T1bN0 esophageal squamous cell carcinoma after definitive therapies: supplementary analysis of the JCOG trial: JCOG0502. J Gastroenterol, 57(7):455-463,2022.7
- Aritake T, Ouchi A, Komori K, Kinoshita T, Sato Y, Nakamura R, Takanari K, Taniguchi H, Muro K, Kato S, Abe T, Ito S, Shimizu Y:Colon cancer with extensive invasion of the abdominal wall treated with neoadjuvant chemotherapy and a free anterolateral thigh flap. Surg Case Rep, 8(1):159,2022.8
- Okada M, Kato K, Cho BC, Takahashi M, Lin CY, Chin K, Kadowaki S, Ahn MJ, Hamamoto Y, Doki Y, Yen CC, Kubota Y, Kim SB, Hsu CH, Holtved E, Xynos I, Matsumura Y, Takazawa A, Kitagawa Y:Three-year follow-up and response-survival relationship of nivolumab in previously treated patients with advanced esophageal squamous cell carcinoma (ATTRACTION-3). Clin Cancer Res, 28(15):3277-3286,2022.8
- Pavlakis N, Tincknell G, Lim LE, Muro K, Obermannova R, Lorenzen S, Chua YJ, Jackson C, Karapetis CS, Price T, Chantrill L, Segelov E, Lordick F:European-Australasian consensus on the management of advanced gastric and gastro-oesophageal junction cancer: current practice and new directions. Ther Adv Med Oncol, 14,2022.8
- Hino K, Nishina T, Kajiwara T, Bando H, Nakamura M, Kadowaki S, Minashi K, Yuki S, Ohta T, Hara H, Mizukami T, Moriwaki T, Ohtsubo K, Komoda M, Mitani S, Nagashima F, Kato K, Yamada T, Hasegawa H, Yamazaki K, Yoshino T, Hyodo I:Association of ERBB2 Copy Number and Gene Coalterations With Trastuzumab Efficacy and Resistance in Human Epidermal Growth Factor Receptor 2-Positive Esophagogastric and Gastric Cancer. JCO Precis Oncol, 6:e2200135,2022.8
- Masuishi T, Nagaoka S, Jin L, Yoshizawa K:A post-marketing safety study of ramucirumab with FOLFIRI in patients with metastatic colorectal cancer. J Gastrointest Oncol, 13(4):1701-1710,2022.8
- Ogata T, Muro K:[Ⅲ. A New Era of Chemotherapy for Advanced Gastric Cancer]. Gan To Kagaku Ryoho, 49(9):942-946,2022.9
- Takahashi T, Ishida K, Emi Y, Sakamoto M, Imura J, Aishima S, Muro K, Uetake H, Oki E, Katayose Y, Yoshida K, Unno M, Hyodo I, Tomita N, Sugihara K, Maehara Y:Pathological Evaluation of Resected Colorectal Liver Metastases: mFOLFOX6 Plus Bevacizumab versus mFOLFOX6 Plus Cetuximab in the Phase II ATOM Trial. Cancers (Basel), 14(18):4392,2022.9
- Kojima T, Hara H, Tsuji A, Yasui H, Muro K, Satoh T, Ogata T, Ishihara R, Goto M, Baba H, Nishina T, Han S, Sakata T, Yatsuzuka N, Doi T, Kato K:First-line pembrolizumab + chemotherapy in Japanese patients with advanced/metastatic esophageal cancer from KEYNOTE-590. Esophagus, 19(4):683-692,2022.10
- Kojima T, Kato K, Hara H, Takahashi S, Muro K, Nishina T, Wakabayashi M, Nomura S, Sato A, Ohtsu A, Doi T:Phase II study of BKM120 in patients with advanced esophageal squamous cell carcinoma (EPOC1303). Esophagus, 19(4):702-710,2022.10
- Sakai SA, Aoshima M, Sawada K, Horasawa S, Yoshikawa A, Fujisawa T, Kadowaki S, Denda T, Matsuhashi N, Yasui H, Goto M, Yamazaki K, Komatsu Y, Nakanishi R, Nakamura Y, Bando H, Hamaya Y, Kageyama SI, Yoshino T, Tsuchihara K, Yamashita R:Fecal microbiota in patients with a stoma decreases anaerobic bacteria and alters taxonomic and functional diversities. Front Cell Infect Microbiol, 12:925444,2022.9
- Nagai S, Nishihara H, Suzuki T, Nishio K, Taniguchi H, Tsuchihara K, Nakamura K, Takamatsu R, Ueno T, Aburatani H, Kohno T, Kohsaka S:Recommendations related to the analytical equivalence assessment of gene panel testing. Cancer Sci, 113(10):3282-3290,2022.10
- Kajiwara T, Nishina T, Nakasya A, Yamashita N, Yamashita R, Nakamura Y, Shiozawa M, Yuki S, Taniguchi H, Hara H, Ohta T, Esaki T, Shinozaki E, Takashima A, Moriwaki T, Denda T, Ohtsubo K, Sunakawa Y, Horita Y, Kawakami H, Kato T, Satoh T, Ando K, Mizutani T, Yasui H, Goto M, Okuyama H, Yamazaki K, Yoshino T, Hyodo I:NOTCH gene alterations in metastatic colorectal cancer in the Nationwide Cancer Genome Screening Project in Japan (SCRUM-Japan GI-SCREEN). J Cancer Res Clin Oncol, 148(10):2841-2854,2022.10
- Kakeji Y, Ishikawa T, Suzuki S, Akazawa K, Irino T, Miyashiro I, Ono H, Suzuki H, Tanabe S, Kadowaki S, Muro K, Fukagawa T, Nunobe S, Wada T, Katai H, Kodera Y; Registration Committee of the Japanese Gastric Cancer Association:A retrospective 5-year survival analysis of surgically resected gastric cancer cases from the Japanese Gastric Cancer Association nationwide registry (2001-2013). Gastric Cancer, 25(6):1082-1093,2022.11
- Pfeiffer P, Lustberg M, Näsström J, Carlsson S, Persson A, Nagahama F, Cavaletti G, Glimelius B, Muro K:Calmangafodipir for Prevention of Oxaliplatin-Induced Peripheral Neuropathy: Two Placebo-Controlled, Randomized Phase 3 Studies (POLAR-A/POLAR-M). JNCI Cancer Spectr, 6(6):pkac075,2022.11
- Muro K:[Lower G. I./Colon and Rectum Cancer Recent Topics in Metastatic Colorectal Cancer]. Gan To Kagaku Ryoho, 49(11):1205-1206,2022.11
- Akazawa N, Itoh N, Morioka H, Ogata T, Ishibana Y, Murakami H, Narita Y:Cholangitis with Sphingobacterium multivorum and Acinetobacter junii bacteremia in a patient with gastric cancer: A case report. J Infect Chemother, 28(10):1419-1423,2022.10
- Kajimoto Y, Honda K, Nozawa K, Mukai M, Teng L, Igarashi A:Use of Anticancer Therapies and Economic Burden Near the End of Life in Japan: Results From Claims Database. JCO Glob Oncol, e2200227,2022.11
- Kakeji Y, Ishikawa T, Suzuki S, Akazawa K, Irino T, Miyashiro I, Ono H, Suzuki H, Tanabe S, Kadowaki S, Muro K, Fukagawa T, Nunobe S, Wada T, Katai H, Kodera Y; Registration Committee of the Japanese Gastric Cancer Association:A retrospective 5-year survival analysis of surgically resected gastric cancer cases from the Japanese Gastric Cancer Association nationwide registry (2001-2013). Gastric Cancer, 25(6):1082-1093,2022.11
- Muro K:[Lower G. I./Colon and Rectum Cancer Recent Topics in Metastatic Colorectal Cancer]. Gan To Kagaku Ryoho, 49(11):1205-1206,2022.11
- Taniguchi H:[A Decentralized Clinical Trial(DCT)Model in Oncology]. Gan To Kagaku Ryoho, 49(11):1225-1228,2022.11
- Shimozaki K, Hirata K, Sato T, Nakamura M, Kato K, Hirano H, Kumekawa Y, Hino K, Kawakami K, Kito Y, Matsumoto T, Kawakami T, Komoda M, Nagashima K, Sato Y, Yamazaki K, Hironaka S, Takaishi H, Hamamoto Y, Muro K:WJOG13219G: The Efficacy and Safety of FOLFOXIRI or Doublet plus Anti-VEGF Therapy in Previously Untreated BRAFV600E Mutant Metastatic Colorectal Cancer: A Multi-Institutional Registry-Based Study (BRACELET Study). Clin Colorectal Cancer, 21(4):339-346,2022.12
- Nakagawa K, Sho M, Fujishiro M, Kakushima N, Horimatsu T, Okada KI, Iguchi M, Uraoka T, Kato M, Yamamoto Y, Aoyama T, Akahori T, Eguchi H, Kanaji S, Kanetaka K, Kuroda S, Nagakawa Y, Nunobe S, Higuchi R, Fujii T, Yamashita H, Yamada S, Narita Y, Honma Y, Muro K, Ushiku T, Ejima Y, Yamaue H, Kodera Y:Clinical practice guidelines for duodenal cancer 2021. J Gastroenterol, 57(12):927-941,2022.12
- Nakagawa K, Sho M, Fujishiro M, Kakushima N, Horimatsu T, Okada KI, Iguchi M, Uraoka T, Kato M, Yamamoto Y, Aoyama T, Akahori T, Eguchi H, Kanaji S, Kanetaka K, Kuroda S, Nagakawa Y, Nunobe S, Higuchi R, Fujii T, Yamashita H, Yamada S, Narita Y, Honma Y, Muro K, Ushiku T, Ejima Y, Yamaue H, Kodera Y:Clinical practice guidelines for duodenal cancer 2021. J Gastroenterol, 57(12):927-941,2022.12
- Shimozaki K, Hirata K, Sato T, Nakamura M, Kato K, Hirano H, Kumekawa Y, Hino K, Kawakami K, Kito Y, Matsumoto T, Kawakami T, Komoda M, Nagashima K, Sato Y, Yamazaki K, Hironaka S, Takaishi H, Hamamoto Y, Muro K:WJOG13219G: The Efficacy and Safety of FOLFOXIRI or Doublet plus Anti-VEGF Therapy in Previously Untreated BRAFV600E Mutant Metastatic Colorectal Cancer: A Multi-Institutional Registry-Based Study (BRACELET Study). Clin Colorectal Cancer, (4):339-346,2022.12
- Watanabe M, Toh Y, Ishihara R, Kono K, Matsubara H, Miyazaki T, Morita M, Murakami K, Muro K, Numasaki H, Oyama T, Saeki H, Tanaka K, Tsushima T, Ueno M, Uno T, Yoshio T, Usune S, Takahashi A, Miyata H; Registration Committee for Esophageal Cancer of the Japan Esophageal Society:Comprehensive registry of esophageal cancer in Japan, 2015. Esophagus, 20(1):1-28,2023.1
- Aoki M, Kadowaki S, Takahashi N, Suzuki T, Oshima K, Ando T, Yamamoto Y, Kawakami K, Kito Y, Matsumoto T, Shimozaki K, Miyazaki Y, Yamaguchi T, Nagase M, Tamura T, Amanuma Y, Esaki T, Miura Y, Akiyoshi K, Baba E, Makiyama A, Negoro Y, Nakashima K, Sugimoto N, Nagashima K, Shoji H, Boku N:Pattern of disease progression during third-line or later chemotherapy with nivolumab associated with poor prognosis in advanced gastric cancer: a multicenter retrospective study in Japan. Gastric Cancer, 26(1):132-144,2023.1
- Watanabe M, Toh Y, Ishihara R, Kono K, Matsubara H, Miyazaki T, Morita M, Murakami K, Muro K, Numasaki H, Oyama T, Saeki H, Tanaka K, Tsushima T, Ueno M, Uno T, Yoshio T, Usune S, Takahashi A, Miyata H:Comprehensive registry of esophageal cancer in Japan, 2015.Registration Committee for Esophageal Cancer of the Japan Esophageal Society. Esophagus, 20(1):1-28,2023.1
- Narita Y, Muro K :[Discrepancy in Gastric Cancer Chemotherapy between the Asia and West]. Gan To Kagaku Ryoho, 50(1):23-29,2023.1
- Kotani D, Oki E, Nakamura Y, Yukami H, Mishima S, Bando H, Shirasu H, Yamazaki K, Watanabe J, Kotaka M, Hirata K, Akazawa N, Kataoka K, Sharma S, Aushev VN, Aleshin A, Misumi T, Taniguchi H, Takemasa I, Kato T, Mori M, Yoshino T:Molecular residual disease and efficacy of adjuvant chemotherapy in patients with colorectal cancer. Nat Med, 29(1):127-134,2023.1
- Muro K :[The Intention of Planning a Special Feature on "Where We Are Now, What the Problems Are, and Where We Are Going with Our Country's Medical Practice Guidelines"]. Gan To Kagaku Ryoho, 50(2):134,2023.2
- Sakamoto Y, Bando H, Nakamura Y, Hasegawa H, Kuwaki T, Okamoto W, Taniguchi H, Aoyagi Y, Miki I, Uchigata H, Kuramoto N, Fuse N, Yoshino T, Ohtsu A:Trajectory for the Regulatory Approval of a Combination of Pertuzumab Plus Trastuzumab for Pre-treated HER2-positive Metastatic Colorectal Cancer Using Real-world Data. Clin Colorectal Cancer, 22(1):45-52,2023.3
- Kataoka K, Yamada T, Taniguchi H, Ikeda M, Yamazaki K, Kanemitsu Y :Corrigendum to "A ctDNA-driven multidisciplinary treatment strategy for resectable colorectal cancer -what surgical oncologists should know" [Eur. J. Surg. Oncol. 48(1) (2022) 1-2]. Eur J Surg Oncol, S0748-7983(23)00420-1,2023.3
- Nakamura Y, Yamashita R, Okamoto W, Komatsu Y, Yuki S, Ueno M, Kato K, Taniguchi H, Kagawa Y, Denda T, Hara H, Esaki T, Moriwaki T, Sunakawa Y, Oki E, Nagashima F, Nishina T, Satoh T, Kawakami H, Yamaguchi K, Ohtsubo K, Kato T, Horita Y, Tsuji A, Yasui H, Goto M, Hamamoto Y, Wakabayashi M, Ikeno T, Shitara K, Bando H, Tsuchihara K, Miki I, Ichiki H, Ohtsu A, Yoshino T:Efficacy of Targeted Trials and Signaling Pathway Landscape in Advanced Gastrointestinal Cancers From SCRUM-Japan GI-SCREEN: A Nationwide Genomic Profiling Program. JCO Precis Oncol, 7:e2200653,2023.3
- Kajimoto Y, Honda K, Suzuki S, Mori M, Tsubouchi H, Nakao K, Azuma A, Shibutani T, Nagao S, Koyanagi T, Kohara I, Tamaki S, Yabuki M, Teng L, Fujiwara K, Igarashi A:Association between financial toxicity and health-related quality of life of patients with gynecologic cancer. Int J Clin Oncol, 28(3):454-467,2023.3
和文論文
- 門脇重憲,:食道がん(周術期).腫瘍内科 vol.29 No.6,科学評論社:653-659,2022.6月
- 中澤泰子、舛石俊樹:がん薬物療法専門医のための模擬テスト147-解答と解説-.腫瘍内科 vol.29 No.6,科学評論社:757-758,2022.6月
- 白石和寛、杉山圭司、室 圭:胃がん(切除不能転移性).腫瘍内科 vol.29 No.6,科学評論社:660-666,2022.6月
- 中澤泰子、舛石俊樹:がん薬物療法専門医のための模擬テスト147-解答と解説-.腫瘍内科 vol.30 No.1,科学評論社:116-120,2022.7月
- 室 圭:消化器がんの薬物療法.日本内科学会雑誌 第111巻 第3号,日本内科学会:469-477,2022.3月 ※昨年度になるが2022年度に載せる
- 中田晃暢、室 圭:進行・再発食道癌に対する薬物療法.食道疾患の診療 臨牀消化器内科8月増刊号 Vol.37 No.9,日本メディカルセンター:163-170,2022.8月
- 吉田和弘、河野浩二、原 浩樹、室 圭:胃がん一次治療の新しい幕開け-がん免疫療法と化学療法の併用-(Key Opinion座談会).GI Cancer Cutting Edge vol.4 No.1,小野薬品工業株式会社 ブリストル・マイヤーズスクイブ株式会社:8-15,2022.7月
- 松原裕樹、室 圭:1.がん治療におけるがん薬物療法の位置づけ(3)大腸がん.臨牀消化器内科vol.37 No.11,日本メディカルセンター:1410-1416,2022.9月
- 本多和典:経済毒性とその対策.腫瘍内科 vol.30 No.3,科学評論社:298-302,2022.9月
- 室 圭:大腸癌大腸癌治療における最近の話題 総括.癌と化学療法 第49巻第11号,癌と化学療法社:1205-1206,2022.11
- 谷口浩也:がん治験におけるDecentralized Clinical Trial(DCT)の実際.癌と化学療法 第49巻第11号,癌と化学療法社:1225-1228,2022.11
- 松原裕樹:大腸癌大腸癌治療における最近の話題 ⅢHER2陽性大腸癌治療の最前線.癌と化学療法 第49巻第11号,癌と化学療法社:1221-1224,2022.11
- 門脇重憲,:名医に聞く医療最前線「胃がん」.確かな病院選びのための地域情報 東海の医療と病院2023年版,中日新聞社:16-17,2022.11
- 若林宗弘、室 圭:大腸癌治療における免疫チェックポイント阻害薬の役割.臨床外科 第78巻第1号,医学書院:43-48,2023.1
- 成田有季哉、室 圭:胃がん化学療法におけるアジアと欧米の違い.癌と化学療法 第50巻 第1号,癌と化学療法社:23-29,2023.1
- 室 圭:患者用ガイドライン作成委員.患者さんのための胃がん治療ガイドライン2023年版,金原出版:2023.2
- 室 圭:04大腸がん.手術数でわかるいい病院2023,朝日新聞出版:134,2023.3
- 児玉紘幸、藤原 豊:Oncologic emergency.腫瘍内科 vol.31 No.2, 科学評論社:221-225,2023.2
- 室 圭:第3版 発刊によせて.あらゆる症例に対応できる!消化器がん薬物療法 第3版,羊土社:3,2023.3
- 緒方貴次:8 胃がん(大腸がん,膵がんを含む)のがん悪液質とその対策.あらゆる症例に対応できる!消化器がん薬物療法 第3版,羊土社:232-234,2023.3
- 松原裕樹:6 BRAF遺伝子変異大腸がんの治療.あらゆる症例に対応できる!消化器がん薬物療法 第3版,羊土社:252-254,2023.3
- 松原裕樹:7 MSI-H大腸がんの治療.あらゆる症例に対応できる!消化器がん薬物療法 第3版,羊土社:255-257,2023.3
- 松原裕樹:8 HER2陽性大腸がんの治療.あらゆる症例に対応できる!消化器がん薬物療法 第3版,羊土社:258-260,2023.3
- 児玉紘幸:10 大腸がんの遺伝子パネル検査の適切な時期とその意義.あらゆる症例に対応できる!消化器がん薬物療法 第3版,羊土社:264-267,2023.3
- 児玉紘幸:11 conversion surgeryの考え方 薬物療法の選択,治療期間,補助化学療法をどうするか?.あらゆる症例に対応できる!消化器がん薬物療法 第3版,羊土社:268-271,2023.3
- 緒方貴次:4 全身薬物療法の早期治療ライン(一次治療,二次治療)の好中球減少にどう対峙すべきか?.あらゆる症例に対応できる!消化器がん薬物療法 第3版,羊土社:281-283,2023.3
- 緒方貴次:5 全身薬物療法の後方治療ライン(FTD/TPI±BEV)の骨髄抑制.あらゆる症例に対応できる!消化器がん薬物療法 第3版,羊土社:284-286,2023.3
- 児玉紘幸:6 レゴラフェニブの副作用をどうマネジメントすべきか?特に手足症候群,肝機能障害.あらゆる症例に対応できる!消化器がん薬物療法 第3版,羊土社:287-291,2023.3
- 児玉紘幸:7 BRAF阻害薬,MEK阻害薬の副作用とその対策.あらゆる症例に対応できる!消化器がん薬物療法 第3版,羊土社:292-295,2023.3
- 緒方貴次:5 虫垂がん.あらゆる症例に対応できる!消化器がん薬物療法 第3版,羊土社:470-472,2023.3
- 松原裕樹:6 消化管NEC.あらゆる症例に対応できる!消化器がん薬物療法 第3版,羊土社:473-475,2023.3
- 若林宗弘、谷口浩也:がん診療における血液循環腫瘍DNAの役割.腎と透析 第94巻 第3号,東京医学社:456-459,2023.3
- 門脇重憲:臓器別がんの薬物療法9.大腸がん・肛門がん.がんの最新の薬物療法2023-2024,南江堂:143-149,2023.3
学会発表
- 室圭(演者):胃癌薬物療法の新展開.第43回癌免疫外科研究会,モーニングセミナー,京都,2022.5月
- 室圭(演者):胃癌薬物療法の新展開. 第8回日本臨床外科学会福島県支部学術集会教育講演.ランチョンセミナー.福島.2022.5月
- Kagawa Y, Kotani D, Bando H, Takahashi N, Hamaguchi T, Kanazawa A, Kato T, Ando K, Satake H, Shinozaki E, Sunakawa Y, Takashima A, Yamazaki K, Yuki S, Nakajima H, Nakamura Y, Wakabayashi M, Taniguchi H, Ohta T, Yoshino T: Plasma RAS dynamics and anti-EGFR rechallenge efficacy in patients with RAS/BRAF wild-type metastatic colorectal cancer: REMARRY and PURSUIT trials. ASCO 2022, Poster Discussion Session , Chicago , 2022. 6月#3518
- Yuki S, Sunakawa Y, Yamazaki K, Shirasu H, Taniguchi H, Masuishi T, Shiozawa M, Bando H, Nishina T, Yasui H, Ohta T, Takahashi N, Denda T, Esaki T, Kawakami H, Satake H, Takashima A, YukikoAbe Y, Nomura S, Yoshino T: Analysis of plasma angiogenesis factors on the efficacy of first-line (1L) chemotherapy (chemo) combined with biologics in RAS wild-type metastatic colorectal cancer (mCRC): Results from GI-SCREEN CRC Ukit study. ASCO 2022, Poster Session , Chicago , 2022. 6月#3530
- Caughey B.A, Umemoto K, Green M, Ikeda M, D’Anna R, Ueno M, Niedzwiecki D, Taniguchi H, Walden D, Komatsu Y, Zhou K. I, Esaki T, Ramaker R, Denda T, Datto M, Bando H, Bekaii-Saab T.S, Yoshino T, Strickler J.H, Nakamura Y: Identification of an optimal circulating tumor DNA (ctDNA) shedding threshold to detect actionable driver mutations in colorectal and pancreatic adenocarcinoma. ASCO 2022, Poster Session , Chicago , 2022. 6月#3571
- Takahashi H, Caughey B.A, Umemoto K, Green M, Nakamura Y, Datto M, Ueno M, Walden D, Esaki T, Oliver T, Komatsu Y, Mizuno N, Oki E, Taniguchi H, Bando H, Morizane C, Yoshino T, Strickler J.H, Ikeda M, Bekaii-Saab T.S: Clinical impact of MAPK pathway alterations in advanced biliary tract cancer (BTC):SCRUM-Japan GOZILA and COLOMATE international collaboration. ASCO 2022, Poster Session , Chicago , 2022. 6月#4086
- Honma Y, Monden N, Yamazaki K, Kano S, Satake H, Kadowaki S, Nagao T, Nakatogawa T, Fujii K, Koroki Y, Aoyama J, Ouchi S, Ogawa T, McCarthy S, Brookman-May S.D, Mundle S, Li J, Tada Y: Yatagarasu: A single-arm, open-label, phase 2 study of apalutamide (APA) plus goserelin (GOS) for patients (pts) with far locally advanced or recurrent/metastatic (fLA/RM) and androgen receptor (AR)-expressing salivary gland carcinoma (SGC). ASCO 2022, Poster Session , Chicago , 2022. 6月#6076
- Smyth E.C, Chao J, Muro K, Yen P, Yanes R.E, Zahlten-Kumeli A, Rha S.Y: Trial in progress: Phase 3 study of bemarituzumab + mFOLFOX6 versus placebo + mFOLFOX6 in previously untreated advanced gastric or gastroesophageal junction(GEJ) cancer with FGFR2b overexpression (FORTITUDE-101). ASCO 2022, Poster Session , Chicago , 2022. 6月#TPS4164
- Yoshino T, Watanabe J, Shitara K, Yasui H, Ohori H, Shiozawa M, Yamazaki K, Oki E, Sato T, Naitoh T, Komatsu Y, Kato T, Hihara M, Soeda J, Yamamoto K, Akagi K, Ochiai A, Uetake H, Tsuchihara K, Muro K: Panitumumab (PAN) plus mFOLFOX6 versus bevacizumab (BEV) plus mFOLFOX6 as first-line treatment in patients with RAS wild-type (WT) metastatic colorectal cancer (mCRC): Results from the phase 3 PARADIGM trial. ASCO 2022, Plenary Session , Chicago , 2022. 6月# LBA1
- Muro K,Watanabe J,Shitara K,Yamazaki K, Ohori H, Shiozawa M,Yasui H,Oki E, Sato T, Naitoh T, Komatsu Y, Kato T, Hihara M, Soeda J,Yamamoto K, Akagi K, Ochiai A, Uetake H, Yoshino T: First-line panitumumab versus bevacizumab in combination with mFOLFOX6 for RAS wild-type metastatic colorectal cancer: PARADIGM trial results. ESMO-GI 2022,Late-breaking abstract presentation Oral Presentation,Barcelona, 2022. 6月LBA O-10
- Muro K(Discussant): Nivolumab (NIVO) plus chemotherapy (chemo) or ipilimumab (IPI) vs chemo as first-line treatment for advanced esophageal squamous cell carcinoma (ESCC): Expanded efficacy and safety analyses from CheckMate 648. ESMO-GI 2022, Oral Presentation,Barcelona, 2022. 6月O-3
- Muro K(Discussant): Trifluridine/tipiracil (TAS-102) with or without bevacizumab in patients with pretreated metastatic esophago-gastric adenocarcinoma (mEGA): A Danish randomized trial (LonGas). ESMO-GI 2022, Oral Presentation,Barcelona, 2022. 6月O-4
- Muro K(Discussant): Co-occurring HER2 and PD-L1 expression in patients with HER2-positive trastuzumabrefractory gastric cancer (GC)/gastroesophageal junction adenocarcinoma (GEJA): Biomarker analysis from the trastuzumab deruxtecan (T-DXd) DESTINY-Gastric03 trial. ESMO-GI 2022, Short Oral Presentation,Barcelona, 2022. 6月SO-7
- Muro K(Discussant): Soluble programmed cell death ligand 1 associated with clinical outcome in gastric cancer patients treated with nivolumab:Blood based biomarker analysis of DELIVER trial (JACCRO-GC08AR). ESMO-GI 2022, Short Oral Presentation,Barcelona, 2022. 6月SO-8
- Muro K(Discussant): The impact of COVID-19 on diagnosis,stage and treatment of esophageal and gastric cancer. ESMO-GI 2022, Poster Discussion,Barcelona, 2022. 6月PD-1
- Muro K(Discussant): EMERGE: A multi-centre, non-randomised,single-arm phase II study investigating domatinostat plus avelumab in patients with previously treated advanced mismatch repair-proficient oesophagogastric and colorectal adenocarcinoma. ESMO-GI 2022, Poster Discussion,Barcelona, 2022. 6月PD-2
- Muro K(Discussant): Phase 1 trial of vibostolimab plus pembrolizumab for PD-1/PD-L1 inhibitornaive advanced gastric cancer: The KEYVIBE-001 trial. ESMO-GI 2022, Poster Discussion,Barcelona, 2022. 6月PD-3
- Muro K(Speaker): Checkpoint Inhibition in Gastric Cancer. ESMO-GI 2022, Session VIII: Esophageal and Gastric Cancers ,Barcelona, 2022. 6月
- Kagawa Y, Kotani D, Bando H, Takahashi N, Horita Y, Kanazawa A,Kato T,Ando K,Satake H, Shinozaki E,Sunakawa Y,Takashima A, Yamazaki K,Yuki S, Nakajima H, Nakamura Y, Wakabayashi M, Taniguchi H, Ohta T,Yoshino T: Plasma RAS dynamics and efficacy of anti-EGFR rechallenge in patients with RAS/BRAF wild-type metastatic colorectal cancer: REMARRY and PURSUIT trials. ESMO-GI 2022, Poster Discussion,Barcelona, 2022. 6月PD-13
- Sunakawa Y,Inagaki C, Yuki S, Shiozawa M,Tsuji A, Matoba R, Inoue E, Muro K, Ichikawa W: An observational/translational study of BRAF inhibitor combination therapy for BRAF-mutant metastatic colorectal cancer including biomarker research: BEETS trial (JACCROCC-18). ESMO-GI 2022, Poster Presentation,Barcelona, 2022. 6月P-57
- Matsuoka H, Narita Y, Misumi T, Sakamoto Y, Kawakami T, Tanioka H, Matsushima T, Miwa H, Shoji H, Ishiguro A, Fushida S, Miura K, Yamada T, Shinozaki K, Mizukami T, Moriwaki T, Mitani S, Nakamura M, Muro K, Nishina T: Impacts of salvage chemotherapy after nivolumab therapy (NIVO) A REVIVE substudy.ESMO-GI 2022, Poster Presentation,Barcelona, 2022. 6月P-61
- Masuishi T, Bando H, Satake H, Kotani D, Hamaguchi T, Shiozawa M, Ikumoto T, Kagawa Y, Yasui H, Moriwaki T, Kawakami H, Boku S, Oki E, Komatsu Y, Taniguchi H, Muro K, Kotaka M, Yamazaki K, Misumi T, Yoshino T, Kato T, Tsuji A: A multicenter randomized phase II study comparing CAPOXIRI plus bevacizumab and FOLFOXIRI plus bevacizumab as the first-line treatment for metastatic colorectal cancer: A safety analysis of the QUATTRO-II study. ESMO-GI 2022, Poster Presentation,Barcelona, 2022. 6月P-80
- Yamazaki K,Taniguchi H, Masuishi T, Kawakami T, Onozawa Y, Honda K, Tsushima T, Hamauchi S, Mori K, Yasui H, Muro K: Bevacizumab, irinotecan and biweekly trifluridine/tipiracil for pretreated metastatic colorectal cancer: MODURATE,a phase Ib study. ESMO-GI 2022, Poster Presentation,Barcelona, 2022. 6月P-95
- Hara H, Masuishi T, Ando T, Kawakami T, Yamamoto Y, Sugimoto N, Shiraishi K, Esaki T, Negoro Y, Tsuzuki T, Sawai H, Nakamura M, Inagaki T, Shinohara Y, Kawakami H, Kawakami K, Katsuya H,Maeda O,Fujita Y,Yoshimura K, Nakajima T, Muro K: A multicenter phase II study of mFOLFOX6 in advanced gastric cancer patients with severe peritoneal metastases:WJOG10517G. ESMO-GI 2022, Poster Presentation,Barcelona, 2022. 6月P-99
- Okita Y, Tsuji A, Watanabe T, Satake H, Goto M, Yasui H, Nakamura M, Sagawa T, Kataoka K, Shiozawa M, Sunakawa Y, Ota H, Kotaka M, Miwa K, Kobayashi Y, Okuyama H, Kochi M, Masuishi T, Takeuchi M, Ichikawa W, Fujii M: Efficacy of 2nd-line ramucirumab (RAM) plus FOLFIRI for RAS wild-type metastatic colorectal cancer (mCRC) by prior regimen: Subgroup analysis of the JACCRO CC-16. ESMO-GI 2022, Poster Presentation,Barcelona, 2022. 6月P-107
- Muro K, Bruce J.Y, Baranda J, Campbell M, Wu C, Gorla S, Braiteh F: EV-202: An open-label, multicenter, phase 2 study of enfortumab vedotin in patients with previously treated locally advanced or metastatic solid tumors, including multiple gastroesophageal cancer cohorts. ESMO-GI 2022, Poster Presentation,Barcelona, 2022. 6月P-134
- Taniguchi H, Kuboki Y, Watanabe J, Terazawa T, Kawakami H, Yokota M, Nakamura M, Kotaka M, Sugimoto N, Ojima H, Oki E, Kajiwara T, Moriwaki T, Takayama T, Denda T, Tamura T, Sunakawa Y, Ishihara S, Nakajima T, Morita S, Shirao K, Yoshino T: Biomarker analysis using plasma angiogenesis factors in the TRUSTY study: A randomized phase 2/3 study of trifluridine/tipiracil plus bevacizumab as second-line treatment for metastatic colorectal cancer. ESMO-GI 2022, Short Oral Presentation, Barcelona, 2022. 6月SO-19
- 室圭(演者): Challenges and future prospects of perioperative chemotherapy for dMMR/MSI-H gastric cancer dMMR/MSI-H胃癌に対する周術期化学療法の課題と今後の展望. 第77回日本消化器外科学会総会, コンセンサスミーティング, 横浜, 2022.7月
- 室圭(座長): 大腸がん個別化医療~Precision Medicineの現在地~. 第97回大腸癌研究会学術集会. ランチョンセミナー, 東京, 2022年7月
- Kawakami H, Kadowaki S, Hirata K, Tsuda M, Esaki T, Sugimoto N, Makiyama A, Machida N, Hirano H, Hara H, Kawakami T, Okamoto W, Yabusaki H, Komatsu Y, Hironaka S, Muro K: Investigator-initiated phase II study of nivolumabplus low-dose ipilimumab as first-line therapy for microsatellite instability—high advanced gastric or esophagogastric junction cancer (NO LIMIT, WJOG13320G/CA209-7W7). ESMO 2022, Poster session, Paris, 2022.9月 1259TiP
- Kito Y, K Yamazaki K, Shoji H, Yamada T, Tsushima T, Mitani S, Shiraish Ki, Yasui H, Hara H, Shimozaki K, Esaki T, Shimokawa H, Kajiura S, Masuishi T, Baba E, Yoshimura K, Kawakami H, Hironaka S, Muro K: Randomized phase II study of FOLFIRI plus ramucirumab versus FOLFOXIRI plus ramucirumab as first-line treatment for metastatic colorectal cancer: WJOG9216G (RECAST). ESMO 2022, Poster session, Paris, 2022.9月 389P
- Muro K, Watanabe J, Shitara K, Yamazaki K, Ohori H, Shiozawa M, Yasui H, Oki E, Sato T, Naitoh T, Komatsu Y, Kato T, Hihara M, Soeda J, Yamamoto K, Akagi K, Ochiai A, Uetake H, Tsuchihara K, Yoshino T: Early tumor shrinkage (ETS) and depth of response (DpR) analyses in metastatic colorectal cancer (mCRC) treated with first-line mFOLFOX6 plus panitumumab (PAN) or bevacizumab (BEV): Results from the phase III PARADIGM trial. ESMO 2022, Poster session, Paris, 2022.9月 388P
- Sunakawa Y, Inoue E, Sakamoto Y, Kawabata R, Ishiguro A, Akamaru Y, Kito Y, Takahashi M, Matsuyama J, Yabusaki H, Makiyama A, Suzuki T, Tsuda M, Yasui H, Kawakami H, Nakajima T.E., Muro K, Matoba R, Ichikawa W, Fujii M: Final analysis of clinical outcomes in the DELIVER trial: Observational study of nivolumab treatment in advanced gastric cancer (JACCRO GC-08). ESMO 2022, Poster session, Paris, 2022.9月 1224P
- Muro K, Kato K, Chin K, Nishino K, Satouchi M, Watanabe Y, Kawakami H, Tsushima T, Hirai H, Chisamore M, Kojima T: Phase Ib study of futibatinib plus pembrolizumab in patients with advanced or metastatic solid tumors: Tolerability results and antitumor activity in esophageal carcinoma. ESMO 2022, Poster session, Paris, 2022.9月 1241P
- Shoji H, Boku N, Kudo-Saito C, Nagashima K, Tsugaru K, Takahashi N, Kawakami T, Amanuma Y, Wakatsuki T, Okano N, Narita Y, Yamamoto Y, Kizawa R, Imazeki H, Aoki K, Muro K: Profiling of myeloid cells associated with prognosis in nivolumab monotherapy for advanced gastric cancer (WJOG10417GTR study). ESMO 2022, Poster session, Paris, 2022.9月 1217P
- Boku N, Kudo-Saito C, Imazeki H, Shoji H, Tsugaru K, Takahashi N, Kawakami T, Amanuma Y, Wakatsuki T, Okano N, Narita Y, Yamamoto Y, Kizawa R, Nagashima K, Aoki K, Muro K: Prognostic impact of myeloid subsets in nivolumab monotherapy for advanced gastric cancer (WJOG10417GTR study). ESMO 2022, Poster session, Paris, 2022.9月 1244P
- Masuishi T, Kuboki Y, Fakih M.G., Strickler J.H., Furqan M, Kim E.J., Cardona P, Tran Q, Chan E, Hong D.S.: Trial in progress: A phase Ib study of sotorasib, a selective KRAS G12C inhibitor, in combination with panitumumab and FOLFIRI in treatment naïve and previously treated metastatic colorectal cancer (CodeBreaK 101). ESMO 2022, Poster session, Paris, 2022.9月 444TiP
- Yuki S, Yamazaki K, Sunakawa Y, Taniguchi H, Masuishi T, Shiozawa M, Bando H, Nishina T, Yasui H, Ohta T, Takahashi N, Denda T, Yoshida K, Kato T, Oki E, Okugawa Y, Ebi H, Abe Y, Nomura S, Yoshino T: Analysis of plasma angiogenesis factors on the efficacy of 2nd-line (2L) chemotherapy (chemo) combined with angiogenesis inhibitors (AIs) in metastatic colorectal cancer (mCRC): Results from GI-SCREEN CRC Ukit study. ESMO 2022, Poster session, Paris, 2022.9月 349P
- Sawada K , Nitta H , Nakamura Y , Okamoto W , Taniguchi H, Komatsu Y , Hara H ,Kato T , Nishina T, Ohta T , Esaki T, Yoshino T , Fujii S: HER2 intratumoral genetic and non-genetic heterogeneity in metastatic colorectal cancer. ESMO 2022, Poster session ,Paris, 2022.9月 1705P
- Bando H, Kumagai S, Kotani D, Saori M, Habu T, Tsushima T, Hara H, Kadowaki S, Kato K, Chin K, Yamaguchi K, Kageyama S, Hojo H, Nakamura M, Tachibana H, Wakabayashi M, Fukutani M, Fuse N, Nishikawa H, Kojima T: A multicenter phase II study of atezolizumab monotherapy following definitive chemoradiotherapy for unresectable locally advanced esophageal squamous cell carcinoma (EPOC1802). ESMO 2022, Poster session ,Paris, 2022.9月 1211P
- Bai L, Chiu C, Kadowaki S, Robert M, Hara H, Hong M.H., Bergamo F, Pernot S, Cunningham D, Lin C, Keam B, Matsumura Y, Enya K, Waxman I, Jin L, Ngo D, Drews U, Mancao C, LeBerre M, Kato K: A phase II study of regorafenib in combination with nivolumab in patients with recurrent or metastatic solidtumors: Results of the ESCC cohort. ESMO 2022, Poster session ,Paris, 2022.9月 1209P
- Oka H, Nagashima K, Nishi H, Kumai Y, Iijima H, Okami K, Shimizu Y, Kano S, Ito K, Yamazaki T, Takahashi H, Oridate N, Yokota T, Koyama T, Kiyota N, Kato K, Kadowaki S, Honma Y: Clinical outcomes in patients with recurrent ormetastatic head and neck squamous cell carcinoma afterfailure of platinum and nivolumab: A multicenter retrospectivestudy. ESMO 2022, Poster session ,Paris, 2022.9月 679P
- Doi T, Patel M, Falchook G.S., Koyama T, Friedman C.F., Piha-Paul S, Gutierrez M.E., Abdul-Karim R, Awad M, Adkins D.R., Takahashi S, Kadowaki S, Cheng B, Ikeda N, Laadem A, Yoshizuka N, Qian M, Dosunmu O, Arkenau H, Johnson M.L.: DS-7300 (B7-H3 DXd antibody-drug conjugate [ADC]) shows durable antitumor activity in advanced solid tumors: Extended follow-up of a phase I/II study. ESMO 2022, Proffered Paper session: Developmental therapeutics ,Paris, 2022.9月 4530
- 室圭(司会):食道癌・接合部癌における免疫チェックポイント 阻害剤の意義と課題. 第76回日本食道学会学術集会,シンポジウム,東京,2022.9月SY1
- 室圭(演者):食道癌のエビデンス(KN 590, CM 648/577)の紹介とガイドラインの推奨. 第76回日本食道学会学術集会,シンポジウム,東京,2022.9月指定講演1
- 松原裕樹(演者):切除不能食道扁平上皮癌に対するニボルマブにおけるCPS/TPSの影響. 第76回日本食道学会学術集会,シンポジウム,東京,2022.9月SY7-01
- 室圭(司会):根治的CRT後のサーベイランスはどうするか?. 第76回日本食道学会学術集会,ワークショップ,東京,2022.9月WS7
- 熊西亮介(演者):Optimal primary prophylaxis for febrile neutropenia during neoadjuvant DCF. 第76回日本食道学会学術集会,優秀演題口演,東京,2022.9月EP2-06
- 門脇重憲(座長):がん-化学療法5. 第76回日本食道学会学術集会,ポスター,東京,2022.9月P24
- 室圭(座長):食道癌における術後ニボルマブ補助療法を考える. 第76回日本食道学会学術集会,ランチョンセミナー,東京,2022.9月LS5
- 谷口浩也(座長):大腸がん(1). 第81回日本癌学会学術総会,ポスター,横浜,2022.9月 P14-26
- 室圭(講師):胃癌薬物療法の基本的な考え方と最新情報.日本消化器病学会北陸支部第47回教育講演会,口演,金沢,2022.10月
- 安藤正志(演者):医学系研究の倫理について. 第60 回日本癌治療学会学術集会,教育セッション,神戸,2022.10月ESS4
- Watanabe J, Muro K , Shitara K, Yamazaki K, Ohori H, Shiozawa M,Yasui H, Oki E, Sato T, Naitoh T, Komatsu Y ,Kato T ,Hihara M,Soeda J, Yamamoto K, Akagi K, Ochiai A, Uetake H, Tsuchihara K,Yoshino T: Impact of R0 resection (R0) on overall survival (OS) in metastatic colorectal cancer (mCRC) treated with first-line mFOLFOX6 plus panitumumab (PAN) or bevacizumab (BEV): results from PARADIGM. 第60 回日本癌治療学会学術集会, 会長企画シンポジウム,神戸,2022.10月SSY3-1-4
- Yoshino T, Watanabe J ,Shitara K, Yamazaki K, Ohori H, Shiozawa M, Yasui H, Oki E, Sato T, Naitoh T, Komatsu Y, Kato T, Hihara M, Soeda J, Yamamoto K, Akagi K, Ochiai A, Uetake H, Tsuchihara K,Muro K: Panitumumab (PAN) plus mFOLFOX6 versus be-vacizumab (BEV) plus mFOLFOX6 as first-line treatment for RAS wild-type (WT) metastatic colorectal cancer (mCRC): Results from the phase 3 PARADIGM trial. 第60 回日本癌治療学会学術集会, アンコールセッション,神戸,2022.10月 EN3-5
- 松原淳一、向井久美、近藤知大、鹿毛秀宣、織田克利、工藤亮、池田貞勝、衣斐寛倫、室圭、林龍二、徳留なほみ、山本信之、武藤学: 化学療法未施行切除不能進行がんに対する遺伝子パネル検査の有用性を評価する臨床研究. 第60 回日本癌治療学会学術集会, 口演,神戸,2022.10月 O48-1
- 高山浩一、仲山真弘、本田主税、遠藤俊充、室圭: アナモレリンのリアルワールドデータ-特定使用成績調査の中間解析. 第60 回日本癌治療学会学術集会, 口演,神戸,2022.10月 O77-1
- 平成人、南博信、岩田広治、弦間昭彦、室圭、市原英基、加藤恭子、木川雄一郎、清田尚臣、久保田馨、建石良介、中田晃暢、中村圭一郎、成田有季哉、堀田勝幸: 切除不能進行/転移・再発固形癌に対するePROモニタリングの有用性を検証する比較試験. 第60 回日本癌治療学会学術集会, 口演,神戸,2022.10月 O48-3
- 森脇俊和、坂東英明、佐竹悠良、小谷大輔、濱口哲弥、沖英次、小松嘉人、谷口浩也、室圭、小高雅人、山﨑健太郎、三角俊裕、吉野孝之、辻晃仁、加藤健志:切除不能大腸癌に対するCAPOXIRI+BEV vs FOLFOXIRI+BEV:QUATTRO-II 試験の安全性解析. 第60 回日本癌治療学会学術集会, 口演,神戸,2022.10月 O53-3
- 谷岡洋亮、成田有季哉、三角俊裕、坂本康寛、松岡宏、川上武志、松島知広、三輪洋人、庄司広和、水上拓郎、仁科智裕、森脇俊和、三谷誠一郎、中村路夫、室圭: ニボルマブ治療不応・不耐後の化学療法を行った進行胃癌患者の予後解析:REVIVE study. 第60 回日本癌治療学会学術集会, eポスター,神戸,2022.10月 P75-5
- 岡田真央、杉山圭司、白石和寛、下嵜啓太郎、山本駿、松原裕樹、古田光寛、廣瀬優、小森梓、土橋賢司、舛石俊樹、山本祥之、筑木隆雄、平田賢郎: 進行食道扁平上皮癌に対するFOLFOX を用いた緩和的化学放射線療法. 第60 回日本癌治療学会学術集会, eポスター,神戸,2022.10月 P78-5
- Ando K, Nakamura Y, Kotani D, Misumi T, Hamabe A, Taniguchi H, Watanabe J ,Yamazaki K, Okita K ,Kotaka M,Alexey A、Takemasa I, Kato T, Oki E, Yoshino T: Molecular residual disease may be a strong predictor for recurrence in rectal cancer with upfront surgery: result from the observational GALAXY study in CIRCULATE-Japan project. 第60 回日本癌治療学会学術集会, 会長企画シンポジウム ,神戸,2022.10月 SSY3-1-3
- 青木優、中村能章、谷口浩也、小松嘉人、砂川優、塩澤学、太田高志、江崎泰斗、山﨑健太郎、佐竹悠良、松橋延壽、後藤昌弘、賀川義規、仁科智裕、吉野孝之: 進行胃癌・大腸癌における治療前の血中循環腫瘍DNA 検出の臨床的妥当性第60 回日本癌治療学会学術集会, プレナリーセッション,神戸,2022.10月 PS-1
- 渡邉純、久保木恭利、寺澤哲志、川上尚人、横田満、中村将人、小髙雅人、沖英次、砂川優、石原聡一郎、谷口浩也、中島貴子、森田智視、白尾國昭、吉野孝之: TRUSTY 試験における血管新生関連遺伝子とFTD/TPI+BEV の有効性の検討. 第60 回日本癌治療学会学術集会, アンコールセッション,神戸,2022.10月 EN3-1
- 太田高志、山崎健太郎、賀川義規、小谷大輔、坂東英明、加藤健志、沖英次、篠崎英司、砂川優、結城敏志、中島裕理、中村能章、若林将史、谷口浩也、吉野孝之: RAS/BRAF 野生型進行再発大腸癌患者における血中バイオマーカーのダイナミクスと抗EGFR抗体リチャレンジの有効性。REMARRY 試験/PURSUIT 試験. 第60 回日本癌治療学会学術集会, アンコールセッション,神戸,2022.10月 EN3-3
- 澤田憲太郎、Nitta H、中村能章、岡本渉、谷口浩也、小松嘉人、原浩樹、加藤健志、仁科智裕、太田高志、薦田正人、吉野孝之、藤井誠志: 切除不能大腸がんにおける腫瘍内HER2 不均一性の検討. 第60 回日本癌治療学会学術集会, アンコールセッション,神戸,2022.10月 EN3-4
- Fujisawa T, Sakamoto N, Nakamura Y, Yamashita R, Kuwata T, Bando H, Ishii G, Ueno M, Kadowaki S, Boku S, Makiyama A, Oki E, Yoshino T: Feasibility and Potential Clinical Utility of Multi-omics Analysis for Advanced Solid Tumors: Preliminary Results of the SCRUM-Japan MONSTAR-SCREEN-2. 第60 回日本癌治療学会学術集会, 会長企画シンポジウム,神戸,2022.10月 SSY3-3-3
- Honma Y, Monden N, Yamazaki K, Kano S, Satake H, Kadowaki S, Nagao T. Nakatogawa T, Fuji K, Koroki Y, Aoyama J,Sharon M,Suneel M, Tada Y: Biomarker Analysis with Next Generation Sequencing (NGS) in YATAGARASU, the Phase 2 Study of Apalutamide (APA) plus Goserelin (GOS) for Androgen Receptor (AR)-expressing Salivary Gland Carcinoma (SGC) . 第60 回日本癌治療学会学術集会, 会長企画シンポジウム,神戸,2022.10月 SSY3-4-3
- 熊西亮介、成田有季哉、若林宗弘、児玉紘幸、中田晃暢、中澤泰子、緒方貴次、松原裕樹、本多和典、舛石俊樹、谷口浩也、門脇重憲、安藤正志、田近正洋、室圭: HER2 陽性胃癌に対するT-DXd 治療前のHER2ECD 及びHER2 測定の意義 . 第60 回日本癌治療学会学術集会, 口演,神戸,2022.10月 YOA O70-6
- 室圭、伊澤真木子、渡部亮介、山内麻衣、伊藤雄一郎、濱田昌宏、尾崎正彦、前川慎一郎、青木大輔: 高頻度マイクロサテライト不安定性を有する固形癌でのペムブロリズマブ使用成績調査. 第60 回日本癌治療学会学術集会,領域横断的ワークショップ,神戸,2022.10月 WS6-5
- 室圭、君嶋悠矢、村上裕美、越智研也、松田祐子、武藤学: 日本人胃癌患者に対するニボルマブ+ 化学療法の有効性と安全性に関する観察研究. 第60 回日本癌治療学会学術集会,口演 ,神戸,2022.10月 O23-1
- 室圭(演者):新たな局面を迎えた食道癌薬物療法のいま. 第60 回日本癌治療学会学術集会,学術セミナー ,神戸,2022.10月 LS9
- 室圭(司会):抗がん剤および分子標的治療薬における副作用対策. 第60 回日本癌治療学会学術集会, 領域横断的ワークショップ,神戸,2022.10月 WS16
- 室圭(演者):がん診療へのバイオシミラー導入の意義と今後の対策. 第60 回日本癌治療学会学術集会,学術セミナー ,神戸,2022.10月 LS20
- 室圭(司会:大腸がんの集学的治療. 第60 回日本癌治療学会学術集会, スポンサードシンポジウム,神戸,2022.10月 SPSY1
- 松原裕樹、舛石俊樹、中田晃暢、児玉紘幸、緒方貴次、熊西亮介、中澤泰子、成田有季哉、本多和典、谷口浩也、門脇重憲、安藤正志、田近正洋、細田和貴、室圭: 切除不能大腸癌の1 次治療/後方治療としての抗EGFR 抗体薬に対するHER2 status の影響. 第60 回日本癌治療学会学術集会, eポスター,神戸,2022.10月 P86-2
- 舛石俊樹、長岡創志、金龍、吉澤健一: A post-marketing safety study of ramucirumab with FOLFIRI in Japanese patients with metastatic colorectal cancer. 第60 回日本癌治療学会学術集会, eポスター,神戸,2022.10月 P11-1
- 谷口浩也、舛石俊樹、門脇重憲、成田有季哉、本多和典、安藤正志、小森康司、木下敬史、佐藤雄介、大内晶、室圭: 大腸癌術後補助化学療法におけるshared decision making の実践. 第60 回日本癌治療学会学術集会,臓器別ワークショップ ,神戸,2022.10月 OWS27-8
- 谷口浩也(演者):大腸がんにおける患者中心の医療の実践~理想と現実の間で~.第60 回日本癌治療学会学術集会, イブニングセミナー,神戸,2022.10月 ES25
- 室圭(司会):消化器癌に対する免疫療法の実態.JDDW2022,口演,福岡,2022.10月 統合5
- 室圭(司会):がん悪液質への新提案“エドルミズ”.JDDW2022,ブレックファーストセミナー,福岡,2022.10月 ブレ14
- 室圭(司会):胃癌薬物治療における現状の課題と今後の展望~最適な治療シークエンスを目指して~.JDDW2022,ランチョンセミナー,福岡,2022.10月 ラン46
- 室圭(演者):バイオマーカーに基づく胃癌薬物療法最前線. 第26回日本外科病理学会学術集会,スポンサードセミナー,口演,富山,2022.11月
- 室圭(演者):胃癌治療におけるICIの位置づけと今後の展開. 第35回日本バイオセラピィ学会学術集会総会,シンポジウム,福島,2022.12月 S2-2
- 室圭(座長):胃癌の分子生物学的プロファイルから考える一次治療戦略. 第35回日本バイオセラピィ学会学術集会総会,ランチョンセミナー,福島,2022.12月LS1
- Shimozaki K , Sugiyama K , Koya S , Shiraishi K , Okada M , Matsubara Y ,Furuta M , Hirose S , Komori A , Mitani S , Boku S, Nishimura T ,Tsuchihashi K , Kito Y , Sugaya A, Masuishi T,Matsumoto T ,Tsuzuki T , Yoshii T,Hirata K: Late line FOLFOX therapy after prior cisplatin-based regimen in advanced esophageal squamous cell carcinoma: A multi-institutional retrospective study. ESMO Asia 2022. Poster viewing, Singapore, 2022.12月126P
- Kuboki Y, Yaeger R, Fakih M, Strickler J.H, Masuishi T, Kim E.J, Bestvina C.M, Langer C.J, Krauss J.C, Puri S, Cardona P, Chang E.K, Tran Q, Hong D.S: Sotorasib in combination with panitumumab inrefractory KRAS G12C-mutated colorectal cancer: Safety andeffi cacy for phase Ib full expansion cohort. ESMO Asia 2022. Proffered Paper and Mini Oral session:Gastrointestinal tumours, Singapore, 2022.12月45MO
- 室圭(演者):胃癌薬物療法の基本的な考え方と最新情報. 第48回日本消化器病学甲信越支部教育講演会,教育講演,WEB開催,2022.12月
- Muro K, Iwasa S, Sugimoto N, Kawakami H, Oshima T, Yamaguchi K,Hino K, Hirao M, Kurokawa Y, Kawakami T, Takegawa N, Hara H, Sumiyoshi N, Matsuoka D, Otake Y, Yasudam K, Takase T, Takashima S, Semba T, Kawazoe A: Gastric cancer (GC) cohort of a phase 2 trial of E7389-LF (liposomal formulation of eribulin) in combination with nivolumab. ASCO GI 2023, Poster Session, SanFrancisco, 2023.1月339
- Tsugaru K, Boku N, Kudo-Saito C, Shoji H, Imazeki H, Takahashi N, Kawakami T, Amanuma Y, Wakatsuki T, Okano N, Narita Y, Yamamoto Y, Kizawa R, Nagashima K, Aoki K, Muro K: LAG3-related factors to predict response to nivolumab monotherapy in advanced gastric cancer (WJOG10417GTR study). ASCO GI 2023, Poster Session, SanFrancisco, 2023.1月425
- Mitani S, Kito Y, Kawakami H, Nishina S, Matsumoto T, Tsuzuki T, Shinohara Y, Shimokawa H, Kumanishi R, Ohta T, Kimura S, Kawakami T, Nishina T, Hasegawa H, Akiyoshi K, Chiba Y, Yamazaki K, Hironaka S, Muro K: Multicenter retrospective study of trifluridine/tipiracil (FTD/TPI) plus bevacizumab (BEV) for vulnerable patients with pretreated metastatic colorectal cancer (mCRC): WJOG14520G (TWILIGHT). ASCO GI 2023, Poster Session, SanFrancisco, 2023.1月121
- Shitara K, Muro K, Watanabe J, Yamazaki K, Ohori H, Shiozawa M,Yasui H, Oki E, Sato T, Naito T, Komatsu Y, Kato T, Soeda J, Yamamoto K, Yamashita R, Akagi K, Ochiai A, Uetake H, Tsuchihara K,Yoshino T: Negative hyperselection of patients with RAS wild-type metastatic colorectal cancer for panitumumab: A biomarker study of the phase III PARADIGM trial. ASCO GI 2023, Rapid Abstract Session, SanFrancisco, 2023.1月11
- Shimomura K, Ogata T, Maeda A, Narita Y, Taniguchi H, Murotani K, Tajika M, Hara K, Muro K, Uchida K: Predictors of therapeutic efficacy of anamorelin in patients with gastric, pancreatic, and colorectal cancer. ASCO GI 2023, Poster Session, SanFrancisco, 2023.1月331
- Ooki A, Yamamoto S, Kawakami H, Makino T, Kawazoe A, Masuishi T,Tsushima T, Hirao M, Takegawa N, Hino K, Iwasa S, Hara H,Sumiyoshi N, Matsuoka D, Otake Y, Yasuda K, Takase T, Takashima S,Semba T, Oshima T: The esophageal cancer cohort of a phase 2 trial of E7389-LF (liposomal formulation of eribulin) + nivolumab. ASCO GI 2023, Poster Session, SanFrancisco, 2023.1月337
- Kawakami T, Harada K, Ogata T, Hu Q, Fushiki K, Oshima K,Kadowaki S, Taniguchi H, Muro K, Nakanishi R, Ando K, Nambara S,Yamamura T, Kawamoto Y, Komatsu Y, Oki E, Masuishi T,Yamazaki K, Yuki S: The impacts of initiating regorafenib with reduced dose on treatment outcomes in metastatic colorectal cancer. ASCO GI 2023, Poster Session, SanFrancisco, 2023.1月133
- Takahari D Boku N, Iwasa S, Mizusawa J, Hashimoto T,Yoshikawa T, Kadowaki S, Machida N, Ishido K, Tsuda M,Kinoshita T, Yasuda T, Chin K, Hata H, Ojima T,Yamada Y, Terashima M: The new prognostic index of advanced gastric cancer using the data from JCOG1013. ASCO GI 2023, Poster Session, SanFrancisco, 2023.1月342
- Kotani D, Kagawa Y, Matsubara Y, Bando H, Harada K, Takahashi N,Mihara Y, Nakayama I, Izawa N, Kawakami T, Masuishi T,Hasegawa H, Ohta T, Wakabayashi M, Yoshino T: TRIDENTE trial: A phase II study of rechallenge with encorafenib, binimetinib, and cetuximab in patients with RAS wild-type/BRAF V600E–mutant metastatic colorectal cancer. ASCO GI 2023, Trials in Progress Poster Session , SanFrancisco, 2023.1月TPS264
- Matsubara Y, Bando H, Kotani D, Kagawa Y, Harada K, Osumi H,Izawa N, Kawakami T, Boku S, Matsumoto T, Wakabayashi M,Yoshino T: BAYONET trial: A multicenter phase II trial of staged combination with encorafenib + binimetinib + cetuximab following encorafenib + cetuximab in patients with BRAF V600Emutant metastatic colorectal cancer. ASCO GI 2023, Trials in Progress Poster Session , SanFrancisco, 2023.1月TPS271
- Bando H, Kumagai S, Kotani D, Mishima S, Habu T, Tsushima T, Hara H, Kadowaki S, Kato K, Chin K, Yamaguchi K, Kageyama S,Hojo H, Nakamura M, Tachibana H, Wakabayashi M, Fukui M, Fuse N, Nishikawa H and Kojima T. Investigation of predictive biomarkers in patients treated with atezolizumab monotherapy following definitive chemoradiotherapy for unresectable locally advanced esophageal squamous cell carcinoma (EPOC1802).ASCO GI 2023, Poster Session, San Francisco, 2023.1月
- Muro K (Speaker):Clinical Cancer Research in Japan~the current status and issues~. The 6th International Cancer Research Symposium,Educational Lecture 8,Osaka,2023.1月
- 室圭(演者):令和4 年個人情報保護法改正による生命・医学系指針改正に対する対応①学術研究機関以外における対応. 日本臨床試験学会第14 回学術集会総会,シンポジウム,金沢,2023.2月 S5-1
- 谷口浩也(演者):がん医師主導治験におけるオンライン診療を活用した完全リモート治験の試み. 日本臨床試験学会第14 回学術集会総会,シンポジウム,金沢,2023.2月 S6-5
- 室圭(演者):胃癌薬物療法の最新トピックスと逐次治療の重要性. 第55 回制癌剤適応研究会,シンポジウム(特別講演),鎌倉,2023.2月
- 安藤正志(座長)本多和典(演者):子宮原発ユーイング肉腫の一例. 第6回日本サルコーマ治療研究学会学術集会,口演,神戸,2023.2月MCC-2
- 安藤正志(座長):サルコーマに対する集学的治療:課題と解決策. 第6回日本サルコーマ治療研究学会学術集会,シンポジウム,神戸,2023.2月SY2
- 室圭(座長):薬物療法の新展開. 第95回日本胃癌学会総会,シンポジウム,札幌,2023.2月SY3-2, SY3-3, SY3-4
- Chen L-T, Kang Y-K, Ryu M-H, Oh D-Y ,Oh S.C , Rha S.Y, Lee K-W,大森 健,設樂紘平,櫻本信一,Chung I-J,山口研成,加藤 健 , Sym S.J,門脇重憲,辻 国広, Chen J-S, Bai L-Y,中田貴志,朴 成和: A 3-year follow-up of ATTRACTION-4: nivolumab + chemotherapy in previously untreated gastric cancer. 第95回日本胃癌学会総会,シンポジウム,札幌,2023.2月SY3-2
- 澤田憲太郎,山下理宇,酒井俊輔,吉河 歩,洞澤智至,中村能章,藤澤孝夫,門脇重憲,安井久晃,高橋直樹,町田 望,牧山明資,後藤昌弘,砂川 優,江﨑泰斗,山﨑健太郎,辻 晃仁,吉野孝之: 胃がん患者における腸内細菌叢と臨床的特徴の検討:SCRUM-Japan MONSTAR-SCREEN. 第95回日本胃癌学会総会,シンポジウム,札幌,2023.2月SY4-5
- 室圭(演者):進行胃癌における薬物療法の最新トピックスと治療戦略. 第95回日本胃癌学会総会, スポンサードシンポジウム ,札幌,2023.2月SS1-2
- 庄司広和,工藤千恵,長島健悟,津軽 開,高橋直樹,川上武志,天沼裕介,若槻 尊, 岡野尚弘,成田有季哉,山本祥之,木澤莉香,今関 洋,青木一教,室圭: 切除不能進行胃癌に対するニボルマブ単剤療法における予後と関連するミエロイド系細胞のプロファイリング.第95回日本胃癌学会総会,シンポジウム,札幌, 2023.2月SY6-5
- 川上武志,朴 成和,工藤千恵,庄司広和,長島健悟,津軽 開,高橋直樹,天沼裕介,若槻 尊,岡野尚弘,成田有季哉,山本祥之,木澤莉香,今関 洋,青木一教,室圭: 切除不能進行胃癌に対するニボルマブ単剤療法における予後と関連するミエロイド系細胞のプロファイリング. 第95回日本胃癌学会総会,シンポジウム,札幌, 2023.2月SY6-6
- 室圭(座長):胃癌薬物療法におけるアナモレリンの役割. 第95回日本胃癌学会総会, パネルディスカッション,札幌, 2023.2月PD3-1~6
- 緒方貴次,下村一景,成田有季哉,谷口浩也,前田章光,室谷健太,内田幸作,田近 正洋,室圭: 悪液質を伴う胃癌患者におけるアナモレリンの効果予測因子の検討. 第95回日本胃癌学会総会, パネルディスカッション,札幌, 2023.2月PD3-6
- 成田有季哉(座長):三次化学療法. 第95回日本胃癌学会総会, デジタルポスター ,札幌, 2023.2月DP-011
- 緒方貴次,成田有季哉,若林宗弘,児玉紘幸,中田晃暢, 熊西亮介,中澤泰子,松原裕樹,本多和典,舛石俊樹,谷口浩也, 門脇重憲,安藤正志,室圭:胃癌に対するトリフルリジン/チピラシル±ラムシルマブ療法の後方視的検討.第95回日本胃癌学会総会, デジタルポスター ,札幌, 2023.2月DP-012-8
- 室圭(演者):胃癌薬物療法 最新動向 ~最新データから考えるBest Sequenceの実現~.第95回日本胃癌学会総会, イブニングセミナー ,札幌, 2023.2月ES2
- 舛石俊樹,原 浩樹,安藤孝将,川上武志,山本祥之,杉本直俊,白石和寛,江﨑泰斗,根来裕二,筑木隆雄,澤井寛明,中村将人,稲墻 崇,篠原雄大,川上賢太郎,馬場英司,近藤千紘,吉村健一,中島貴子,室圭: 高度腹膜転移を有する切除不能胃癌に対するmFOLFOX6の第II相試験(WJOG10517G).第95回日本胃癌学会総会, ワークショップ ,札幌, 2023.2月WS4-1
- 室圭(座長):胃癌治療におけるClinical Questionを考える.第95回日本胃癌学会総会, スポンサードシンポジウム,札幌, 2023.2月SS2-1~3
- 松原裕樹,門脇重憲,室圭:胃癌術後再発症例 –後方ラインにおいてConversion手術を行うか–. 第95回日本胃癌学会総会, Virtual Cancer Board ,札幌, 2023.2月VCB2-2
- 緒方貴次(演者):いつ始める?エドルミズの最適な処方タイミングを考える. 第95回日本胃癌学会総会, ランチョンセミナー,札幌, 2023.2月 LS12-2
- 室圭,伊澤真木子,孔 禕彬,山内麻衣,伊藤雄一郎,濱田昌宏,尾崎正彦,前川慎一郎,青木大輔: 高頻度マイクロサテライト不安定性を有する固形癌でのペムブロリズマブ使用成績調査. 第95回日本胃癌学会総会, デジタルポスター ,札幌, 2023.2月DP-083-2
- 室圭(司会):経済毒性の現状と今後. 第20回日本臨床腫瘍学会学術集会, 会長企画シンポジウム,福岡,2023.3月PSY01-1~3
- 本多和典(演者):経済毒性啓発プロジェクト(FT-01). 第20回日本臨床腫瘍学会学術集会, 会長企画シンポジウム,福岡,2023.3月PSY01-1
- 庄司広和,木藤陽介,山﨑健太郎,山田武史,對馬隆浩,三谷誠一郎,白石和寛,安井久晃, 原 浩樹,下嵜啓太郎,江崎泰斗, 下川穂積,筑木隆雄,梶浦新也,舛石俊樹,馬場英司,吉村健一,川上尚人廣中秀一,室圭: WJOG9216G(RECAST):切除不能大腸がん一次治療例に対するFOLFIRI+ラムシルマブとFOLFOXIRI+ラムシルマブのランダム化第II相試験. 第20回日本臨床腫瘍学会学術集会, Oral Session(English Session) ,福岡,2023.3月O4-2
- 三島沙織,小谷大輔,中村能章,坂東英明,三代雅明,浜部敦史,渡邊 純,平田敬治,赤澤直也,片岡幸三,Yeh K-H,Laliotis G,Jurdi A ,Liu M,谷口浩也,竹政伊知朗,加藤健志,森 正樹,吉野孝之,沖 英次: 根治的外科治療後の結腸・直腸がん患者における再発早期予測因子としてのctDNA Dynamics:GALAXY study in the CIRCULATE-Japan. 第20回日本臨床腫瘍学会学術集会, Oral Session(English Session) ,福岡,2023.3月O4-5
- 室圭(司会):Guardant360 CDxを用いたPrecision Oncology. 第20回日本臨床腫瘍学会学術集会,メディカルセミナー,福岡,2023.3月MeS10
- 衣斐寛倫,松原淳一,向井久美,近藤知大,吉岡正博,鹿毛秀宣,織田克利,工藤 亮,池田貞勝,林 龍二,徳留なほみ,山本信之,室圭,武藤 学: 化学療法未施行切除不能進行がんに対するがん遺伝子パネル検査の有用性を評価する前向き臨床研究(先進医療B:FIRST-Dx study). 第20回日本臨床腫瘍学会学術集会, Oral Session(English Session) ,福岡,2023.3月O1-2
- 伊澤直樹,松原裕樹,仁科慎一,塩澤 学,傳田忠道,太田博文,根来裕二,田中千弘,川上尚人,松岡 宏,田邊裕貴,池永雅一,奥田博介,石黒 敦,賀川義規,佐竹悠良, 砂川 優,竹内正弘,市川 度,藤井雅志: RAS変異型切除不能進行・再発大腸癌患者における化学療法後のctDNA中RAS遺伝子変異ステータス:RASMEX study (JACCRO CC-17). 第20回日本臨床腫瘍学会学術集会, Mini Oral Session(English Session) ,福岡,2023.3月MO16-2
- 仁科慎一,木藤陽介,川上尚人,三谷誠一郎,筑木隆雄,松本俊彦,下川穂積,篠原 雄大,熊西亮介,太田高志,木村晋也,川上武志,仁科智裕,長谷川裕子,秋吉宏平,千葉康敬,廣中秀一,山﨑健太郎,室圭: WJOG14520G (TWILIGHT):前治療歴を有するVulnerableな切除不能大腸がん患者に対するFTD/TPI+ベバシズマブの 多施設レトロ研究. 第20回日本臨床腫瘍学会学術集会, Mini Oral Session(English Session) ,福岡,2023.3月MO17-3
- 中村真穂,下嵜啓太郎,平田賢郎,三谷誠一郎,杉山圭司,廣田 玲,有山 寛,杉本 直俊,伊澤直樹,山本祥之,高山歳三,篠原雄大,國枝献治,安藤孝将,長島健悟,佐藤泰憲,山崎健太郎,廣中秀一,高石官均,室圭: BRAF V600E変異型切除不能進行・再発大腸癌に対するFOLFOXIRI+/-Bevacizumab療法とDoublet療法の有効性および安全性に関する後方視的検討.第20回日本臨床腫瘍学会学術集会, Mini Oral Session(English Session) ,福岡,2023.3月MO17-5
- 原田一顕,川上武志,緒方貴次,胡 慶江,伏木邦博,大嶋琴絵,門脇重憲,谷口浩也, 室圭,中西良太,安藤幸滋,南原 翔,山村貴洋,川本泰之,小松嘉人,沖 英次,舛石 俊樹,山﨑健太郎,結城敏志: 切除不能進行再発大腸癌におけるレゴラフェニブの減量開始が治療効果に与える影響. 第20回日本臨床腫瘍学会学術集会, Mini Oral Session(English Session) ,福岡,2023.3月MO17-6
- 白石和寛,杉山圭司,澤井康弥,下嵜啓太郎,岡田真央,松原祐樹,古田光寛,廣瀬 優,小森 梓,三谷誠一郎,朴 将源,西村 尚,土橋賢司,木藤陽介,菅谷明徳,舛石 俊樹,松本俊彦,筑木隆雄,吉井貴子,平田賢郎: 進行再発食道扁平上皮癌の一次治療におけるFOLFOX療法に関する多施設後方視的研究. 第20回日本臨床腫瘍学会学術集会, Mini Oral Session(English Session) ,福岡,2023.3月MO19-2
- 室圭(司会):The Latest Treatment Strategies in Esophageal Cancer. 第20回日本臨床腫瘍学会学術集会, イブニングセミナー ,福岡,2023.3月ES3
- 本多和典(演者):日本人がん患者における経済毒性. 第20回日本臨床腫瘍学会学術集会, モーニングセミナー ,福岡,2023.3月MoS8
- 室圭,仲山真弘,本田主税,町井浩司,遠藤俊充,髙山浩一: がん悪液質患者4,672症例に対するアナモレリンのリアルワールドデータ-特定使用成績調査の中間解析-.第20回日本臨床腫瘍学会学術集会, Oral Session(English Session) ,福岡,2023.3月O8-1
- 児玉紘幸,門脇重憲,若林宗弘,中田晃暢,熊西亮介,中澤泰子,緒方貴次,松原裕樹,本多和典,舛石俊樹,成田有季哉,谷口浩也, 安藤正志,花井信広,室圭: プラチナ抵抗性頭頚部癌に対するパクリタキセル+セツキシマブ vs. ニボルマブの有効性と安全性に関する比較検討. 第20回日本臨床腫瘍学会学術集会, Oral Session(English Session) ,福岡,2023.3月O9-1
- 山口真澄,小原真紀子,上岡亜子,佐野雄三,青木智子,能澤一樹,足立雄太,新津 宏明,井本逸勢,安井久晃,衣斐寛倫: がん専門病院におけるがんゲノムプロファイリング検査結果に基づく治験・臨床試験に関する支援・情報提供と今後の課題. 第20回日本臨床腫瘍学会学術集会, Mini Oral Session(English Session) ,福岡,2023.3月MO45-5
- 朴 将源,酒井俊輔,澤田憲太郎,洞澤智至,吉河 歩,中村能章,藤澤孝夫,山下理宇,小松嘉人,仁科智裕,塩澤 学,西田尚弘,吉田和弘,山﨑健太郎,後藤昌弘,安井久晃,高橋直樹,門脇重憲,傳田忠道: PIK3CA変異大腸癌における腸内細菌の解析 SCRUM-Japan MONSTAR-SCREEN 付随研究. 第20回日本臨床腫瘍学会学術集会, Mini Oral Session(English Session) ,福岡,2023.3月MO32-5
- 本多和典(演者):我が国の後腹膜肉腫に対する治療の現状と課題. 第20回日本臨床腫瘍学会学術集会, シンポジウム(English Session) ,福岡,2023.3月SY15-2
- 室圭(司会):切除不能・進行再発大腸がん治療のup date ~Fit/Vulnerableに分けた治療戦略~. 第20回日本臨床腫瘍学会学術集会, モーニングセミナー,福岡,2023.3月MoS15
- 牧明資,庄司 広和,川上尚人,田村孝雄,杉山圭司,原浩樹,長瀬通隆,西川和男,江崎泰斗,伊澤直樹,中村将人,安藤孝将,三浦裕司,成田有季哉,下川元継,山崎 健太郎,廣中秀一,朴 成和,兵頭一之介,室圭: 高齢者切除不能・再発胃癌に対するS-1単剤療法とS-1/L-OHP併用 (SOX) 療法のランダム化第II相試験. 第20回日本臨床腫瘍学会学術集会, Oral Session(English Session) ,福岡,2023.3月O14-1
- 門脇重憲(ディスカッサント): TCOG1220: a single-arm, phase II trial of BI -754091 and Afatinib for refractory esophageal squamous cell carcinoma. 第20回日本臨床腫瘍学会学術集会, Oral Session(English Session) ,福岡,2023.3月O14-2
- 安藤孝将,舛石俊樹,原 浩樹,川上武志,山本祥之,杉本直俊,白石和寛,江﨑泰斗,根来裕二,筑木隆雄,澤井寛明,中村将人,稲墻 崇,篠原雄大,川上賢太郎,馬場 英司,近藤千紘,吉村健一,中島貴子,室圭: 高度腹膜転移を有する切除不能進行・再発胃癌に対するmFOLFOX6の多施設共同第II相試験(WJOG10517G). 第20回日本臨床腫瘍学会学術集会, Oral Session(English Session) ,福岡,2023.3月O14-3
- 門脇重憲(ディスカッサント): 高度腹膜転移を有する切除不能進行・再発胃癌に対するmFOLFOX6の多施設共同第II相試験(WJOG10517G). 第20回日本臨床腫瘍学会学術集会, Oral Session(English Session) ,福岡,2023.3月O14-3
- 門脇重憲(ディスカッサント): 進行性の消化管間質腫瘍患者を対象とするpimitespib(TAS-116)の第III相試験の最終解析(CHAPTER-GIST-301). 第20回日本臨床腫瘍学会学術集会, Oral Session(English Session) ,福岡,2023.3月O14-4
- 谷崎潤子,米盛 勧,滝口裕一,秋吉宏平,小峰啓吾,小野澤祐輔,佐藤真利子,平田 賢郎,尾上琢磨,大熊遼太朗,堀田洋介,南 博信,本多和典,陶山浩一,伊藤彰彦,千葉康敬,西尾和人,中川和彦,林 秀敏: 原発不明がんに対するニボルマブの安全性と有効性:拡大治験(NivoCUP2, WJOG14620M)の初回結果報告.第20回日本臨床腫瘍学会学術集会, Oral Session(English Session) ,福岡,2023.3月O16-5
- 大木 暁,陳 勁松,坂東英明,熊谷尚悟,小谷大輔,三島沙織,羽部 匠,對馬隆浩,原 浩樹,門脇重憲,加藤 健,山口研成,影山俊一郎,北條秀博,中村匡希,橘 英伸,若林将史,福井 誠,布施 望,西川博嘉,小島隆嗣: 切除不能局所進行食道扁平上皮癌に対する化学放射線療法後のアテゾリズマブの有効性・安全性をみる第II相試験(EPOC1802). 第20回日本臨床腫瘍学会学術集会, Presidential Session (English Session) ,福岡,2023.3月PS4-1
- 室圭,渡邉 純,設樂紘平,山﨑健太郎,大堀久詔,塩澤 学,安井博史,沖 英次,佐藤武郎,内藤 剛, 小松嘉人加藤健志,日原眞弘,添田純平,山本紘司,赤木 究,落合淳志,植竹宏之,土原一哉,吉野孝之: PARADIGM試験における早期腫瘍縮小割合および最大腫瘍縮小割合に関する検討. 第20回日本臨床腫瘍学会学術集会, Presidential Session (English Session) ,福岡,2023.3月PS4-4
- 喜多昭介,下井辰徳,小峰啓吾,安藤正志,有山 寛,沖田南都子,佐立 崚,宋 奈緒子,東 悟史,数見由紀,米盛 勧: 局所進行・再発類上皮肉腫に対するタゼメトスタットの第II相医師主導治験(TAZETTA trial) .第20回日本臨床腫瘍学会学術集会, Oral Session,福岡,2023.3月O17-2
- 室圭(司会):CM 649長期フォローデータを踏まえた1次治療への期待. 第20回日本臨床腫瘍学会学術集会, メディカルセミナー(English Session),福岡,2023.3月MeS27
- 室圭(司会):Progress and future in the treatment of advanced gastric cancer. 第20回日本臨床腫瘍学会学術集会, メディカルセミナー(English Session),福岡,2023.3月MeS27
- 若林宗弘,舛石俊樹,児玉紘幸,中田晃暢,熊西亮介,中澤泰子,緒方貴次,松原裕樹,本多和典,成田 有季哉,谷口浩也, 門脇重憲,安藤正志,田近正洋室 圭: 播種性血管内凝固を合併した切除不能胃癌2次治療におけるパクリタキセル(ナブパクリタキセル)へのラムシルマブ併用の有効性と安全性の検討. 第20回日本臨床腫瘍学会学術集会, ポスターセッション,福岡,2023.3月P46-2
- 谷口浩也(演者):がん臨床試験におけるDCTのあり方、現状と課題. 第20回日本臨床腫瘍学会学術集会, 第10回がん専門CRCのためのアドバンストセミナー,福岡,2023.3月
- 川上武志,朴 成和,工藤千恵,庄司広和,長島健悟,津軽 開,高橋直樹,天沼祐介,若槻 尊,岡野尚弘,成田有季哉,山本祥之,木澤莉香,今関 洋,青木一教,室圭: 切除不能進行胃がんに対するニボルマブ単剤療法におけるミエロイド系サブセットの予後への影響(WJOG10417GTR). 第20回日本臨床腫瘍学会学術集会, Mini Oral Session(English Session) ,福岡,2023.3月MO65-3
- 庄司広和,朴 成和,工藤千恵,長島健悟,津軽 開,高橋直樹,川上武志,天沼裕介,若槻 尊,岡野尚弘,成田有季哉,山本祥之, 木澤莉香,今関 洋,青木一教,室圭: 切除不能進行胃癌に対するニボルマブ単剤療法における予後と関連するミエロイド系細胞のプロファイリング(WJOG10417GTR試験). 第20回日本臨床腫瘍学会学術集会, Mini Oral Session(English Session) ,福岡,2023.3月MO65-4
- 下村一景,緒方貴次,前田章光,成田有季哉,谷口浩也, 室谷健太,藤原 豊,田近 正洋,原 和生,室圭,内田幸作: がん悪液質患者におけるアナモレリンの効果予測因子. 第20回日本臨床腫瘍学会学術集会, Mini Oral Session ,福岡,2023.3月MO70-3
- 門脇重憲(ディスカッサント):高齢者切除不能・再発胃癌に対するS-1単剤療法とS-1/L-OHP併用 (SOX) 療法のランダム化第II相試験.第20回日本臨床腫瘍学会学術集会,Oral Session(English Session),福岡2023.3月O14-1
英文論文
- Kadowaki S, Masuishi T, Ura T, Sugiyama K, Mitani S, Narita Y, Taniguchi H, Muro K: A triplet combination of FOLFOXIRI plus cetuximab as first-line treatment in RAS wild-type, metastatic colorectal cancer: a dose-escalation phase Ib study. Int J Clin Oncol, 26(4):701-707, 2021.4
- Wainberg ZA, Fuchs CS, Tabernero J, Shitara K, Muro K, Van Cutsem E, Bang YJ, Chung HC, Yamaguchi K, Varga E, Chen JS, Hochhauser D, Thuss-Patience P, Al-Batran SE, Garrido M, Kher U, Shih CS, Shah S, Bhagia P, Chao J: Efficacy of Pembrolizumab Monotherapy for Advanced Gastric/Gastroesophageal Junction Cancer with Programmed Death Ligand 1 Combined Positive Score ≥10. Clin Cancer Res, 27(7):1923-1931, 2021.4
- Yoshino T, Uetake H, Funato Y, Yamaguchi Y, Koyama T, Ozawa D, Tajiri M, Muro K: Post-marketing surveillance study of trifluridine/tipiracil in patients with metastatic colorectal cancer. Jpn J Clin Oncol, 51(5):700-706, 2021.4
- Niisato Y, Moriwaki T, Fukuoka S, Masuishi T, Takashima A, Kumekawa Y, Kajiwara T, Yamazaki K, Esaki T, Makiyama A, Denda T, Hatachi Y, Suto T, Sugimoto N, Shimada Y:Clinical Outcomes Following Trifluridine/Tipiracil Treatment for Patients With Metastatic Colorectal Cancer Ineligible for Regorafenib Treatment. Anticancer Res,41(4):2203-2207,2021.4
- Suzuki S, Takahashi A, Ishikawa T, Akazawa K, Katai H, Isobe Y, Miyashiro I, Ono H, Tanabe S, Fukagawa T, Muro K, Nunobe S, Kadowaki S, Suzuki H, Irino T, Usune S, Miyata H, Kakeji Y; Registration Committee of the Japanese Gastric Cancer Association: Surgically treated gastric cancer in Japan: 2011 annual report of the national clinical database gastric cancer registry. Gastric Cancer, 24(3):545-566, 2021.5
- Yamaguchi K, Fuse N, Komatsu Y, Fujii H, Hironaka S, Omuro Y, Muro K, Yasui H, Ueda S, Nishina T, Watanabe M, Ohtsu A: Phase II study of cetuximab plus S-1/cisplatin therapy in Japanese patients with advanced gastric cancer. Jpn J Clin Oncol, 51(6):879-885, 2021.5
- Kagawa Y, Elez E, García-Foncillas J, Bando H, Taniguchi H, Vivancos A, Akagi K, García A, Denda T, Ros J, Nishina T, Baraibar I, Komatsu Y, Ciardiello D, Oki E, Kudo T, Kato T, Yamanaka T, Tabernero J, Yoshino T: Combined Analysis of Concordance between Liquid and Tumor Tissue Biopsies for RAS Mutations in Colorectal Cancer with a Single Metastasis Site: The METABEAM Study. Clin Cancer Res. 27(9):2515-2522, 2021.5
- Suzuki S, Takahashi A, Ishikawa T, Akazawa K, Katai H, Isobe Y, Miyashiro I, Ono H, Tanabe S, Fukagawa T, Muro K, Nunobe S, Kadowaki S, Suzuki H, Irino T, Usune S, Miyata H, Kakeji Y: Surgically treated gastric cancer in Japan: 2011 annual report of the national clinical database gastric cancer registry. Registration Committee of the Japanese Gastric Cancer Association.Gastric Cancer,24(3):545-566, 2021.5
- Kim SY, Lee JS, Kang J, Morita S, Park YS, Sakamoto J, Muro K, Xu RH, Kim TW: Proton Pump Inhibitor Use and the Efficacy of Chemotherapy in Metastatic Colorectal Cancer: A Post Hoc Analysis of a Randomized Phase III Trial (AXEPT). Oncologist, 26(6):e954-e962, 2021.6
- Chao J, Fuchs CS, Shitara K, Tabernero J, Muro K, Van Cutsem E, Bang YJ, De Vita F, Landers G, Yen CJ, Chau I, Elme A, Lee J, Özgüroglu M, Catenacci D, Yoon HH, Chen E, Adelberg D, Shih CS, Shah S, Bhagia P, Wainberg ZA: Assessment of Pembrolizumab Therapy for the Treatment of Microsatellite Instability-High Gastric or Gastroesophageal Junction Cancer Among Patients in the KEYNOTE-059, KEYNOTE-061, and KEYNOTE-062 Clinical Trials. JAMA Oncol, 7(6):895-902, 2021.6
- Oki E, Watanabe J, Sato T, Kagawa Y, Kuboki Y, Ikeda M, Ueno H, Kato T, Kusumoto T, Masuishi T, Yamaguchi K, Kanazawa A, Nishina T, Uetake H, Yamanaka T, Yoshino T: Impact of the 12-gene recurrence score assay on deciding adjuvant chemotherapy for stage II and IIIA/B colon cancer: the SUNRISE-DI study. ESMO Open,6(3):100146,2021.6
- Siena S, Di Bartolomeo M, Raghav K, Masuishi T, Loupakis F, Kawakami H, Yamaguchi K, Nishina T, Fakih M, Elez E, Rodriguez J, Ciardiello F, Komatsu Y, Esaki T, Chung K, Wainberg Z, Sartore-Bianchi A, Saxena K, Yamamoto E, Bako E, Okuda Y, Shahidi J, Grothey A, Yoshino T; DESTINY-CRC01 investigators: Trastuzumab deruxtecan (DS-8201) in patients with HER2-expressing metastatic colorectal cancer (DESTINY-CRC01): a multicentre, open-label, phase 2 trial. Lancet Oncol,22(6):779-789,2021.6
- Nakajima H, Fukuoka S, Masuishi T, Takashima A, Kumekawa Y, Kajiwara T, Yamazaki K, Negoro Y, Komoda M, Makiyama A, Denda T, Hatachi Y, Suto T, Sugimoto N, Enomoto M, Ishikawa T, Kashiwada T, Ando K, Yuki S, Okuyama H, Kusaba H, Sakai D, Okamoto K, Tamura T, Yamashita K, Gosho M, Moriwaki T: Clinical Impact of Primary Tumor Location in Metastatic Colorectal Cancer Patients Under Later-Line Regorafenib or Trifluridine/Tipiracil Treatment. Front Oncol.;11:688709,2021.6
- Nakajima H, Kotani D, Bando H, Kato T, Oki E, Shinozaki E, Sunakawa Y, Yamazaki K, Yuki S, Nakamura Y, Yamanaka T, Yoshino T, Ohta T, Taniguchi H, Kagawa Y. REMARRY and PURSUIT trials: liquid biopsy-guided rechallenge with anti-epidermal growth factor receptor (EGFR) therapy with panitumumab plus irinotecan for patients with plasma RAS wild-type metastatic colorectal cancer. BMC Cancer. 21(1):674,2021.6
- Miura Y, Ando M, Yamazaki K, Hironaka S, Boku N, Muro K, Hyodo I: Time-dependent discrepancies between physician-assessed and patient-reported oxaliplatin-induced peripheral neuropathy in patients with metastatic colorectal cancer who received mFOLFOX6 plus bevacizumab: a post hoc analysis (WJOG4407GSS2). Support Care Cancer, 29(7):3715-3723, 2021.7
- Boku N, Satoh T, Ryu MH, Chao Y, Kato K, Chung HC, Chen JS, Muro K, Kang WK, Yeh KH, Yoshikawa T, Oh SC, Bai LY, Tamura T, Lee KW, Hamamoto Y, Kim JG, Chin K, Oh DY, Minashi K, Cho JY, Tsuda M, Nishiyama T, Chen LT, Kang YK: Nivolumab in previously treated advanced gastric cancer (ATTRACTION-2): 3-year update and outcome of treatment beyond progression with nivolumab. Gastric Cancer, 24(4):946-958, 2021.7
- Mizukami T, Miyaji T, Narita Y,Matsushima T, Ogura T, Miyagaki H, Kawabata R, Horie Y, Kawaguchi T, Muro K, Hara H, Yamaguchi T, E Nakajima T: An observational study on nutrition status in gastric cancer patients receiving ramucirumab plus taxane: BALAST study. Future Oncol, 17(19):2431-2438, 2021.7
- Lin CC, Doi T, Muro K, Hou MM, Esaki T, Hara H, Chung HC, Helwig C, Dussault I, Osada M, Kondo S: Bintrafusp Alfa, a Bifunctional Fusion Protein Targeting TGFβ and PD-L1, in Patients with Esophageal Squamous Cell Carcinoma: Results from a Phase 1 Cohort in Asia. Target Oncol, 16(4):447-459, 2021.7
- Motai R, Sawabe M, Kadowaki S, Sasaki E, Nishikawa D, Suzuki H, Beppu S, Terada H, Hanai N: Clinical impact of weekly paclitaxel plus cetuximab is comparable to the EXTREME regimen for recurrent/metastatic head and neck squamous cell carcinoma. Int J Clin Oncol,26(7):1188-1195,2021.7
- Taniguchi H, Nakamura Y, Kotani D, Yukami H, Mishima S, Sawada K, Shirasu H, Ebi H, Yamanaka T, Aleshin A, Billings PR, Rabinowitz M, Oki E, Takemasa I, Kato T, Mori M, Yoshino T: CIRCULATE-Japan: Circulating tumor DNA-guided adaptive platform trials to refine adjuvant therapy for colorectal cancer, Cancer Sci. 112(7):2915-2920, 2021. 7
- Motai R, Sawabe M, Kadowaki S, Sasaki E, Nishikawa D, Suzuki H, Beppu S, Terada H, Hanai N: Clinical impact of weekly paclitaxel plus cetuximab is comparable to the EXTREME regimen for recurrent/metastatic head and neck squamous cell carcinoma. Int J Clin Oncol. 26(7):1188-1195,2021.7
- Ogata T, Narita Y, Kumanishi R, Nakazawa T, Matsubara Y, Kato K, Nozawa K, Honda K, Masuishi T, Bando H, Kadowaki S, Ando M, Tajika M, Muro K: Clinical Impact of Oral Intake in Second-line or Third-line Chemotherapy for 589 Patients With Advanced Gastric Cancer: A Retrospective Cohort Study. Am J Clin Oncol, 44(8):388-394, 2021.8
- Kato K, Masuishi T, Fushiki K, Nakano S, Kawamoto Y, Narita Y, Tsushima T, Harada K, Kadowaki S, Todaka A, Yuki S, Tajika M, Machida N, Komatsu Y, Yasui H, Muro K, Kawakami T: Impact of tumor growth rate during preceding treatment on tumor response to nivolumab or irinotecan in advanced gastric cancer. ESMO Open, 6(4):100179, 2021.8
- Yamaguchi T, Shimizu J, Hasegawa T, Horio Y, Inaba Y, Hanai N, Muro K, Hida T: Pre-existing interstitial lung disease is associated with onset of nivolumab-induced pneumonitis in patients with solid tumors: a retrospective analysis. BMC Cancer, 21(1):924, 2021.8
- Shitara K, Özgüroğlu M, Bang YJ, Di Bartolomeo M, Mandalà M, Ryu MH, Caglevic C, Chung HC, Muro K, Van Cutsem E, Kobie J, Cristescu R, Aurora-Garg D, Lu J, Shih CS, Adelberg D, Cao ZA, Fuchs CS: Molecular determinants of clinical outcomes with pembrolizumab versus paclitaxel in a randomized, open-label, phase III trial in patients with gastroesophageal adenocarcinoma. Ann Oncol, 32(9):1127-1136, 2021. 9
- Sunami K, Bando H, Yatabe Y, Naito Y, Takahashi H, Tsuchihara K, Toyooka S, Mimori K, Kohsaka S, Uetake H, Kinoshita I, Komine K, Takeda M, Hayashida T, Tamura K, Nishio K, Yamamoto N; Working Group of a Joint Task Force of Three Academic Societies for the Promotion of Cancer Genomic Medicine: Appropriate use of cancer comprehensive genome profiling assay using circulating tumor DNA. Cancer Sci. 2021 Sep;112(9):3911-3917, 2021. 9
- Sugiyama K, Shiraishi K, Sato M, Nishibori R, Nozawa K, Kitagawa C :Salvage Chemotherapy by FOLFIRI Regimen for Poorly Differentiated Gastrointestinal Neuroendocrine Carcinoma.J Gastrointest Cancer, 52(3):947-951, 2021.9
- Oki E, Ando K, Taniguchi H, Yoshino T, Mori M: Sustainable Clinical Development of Adjuvant Chemotherapy for Colon Cancer.Ann Gastroenterol Surg, 9;6(1):37-45, 2021.9
- Adachi Y, Oze I, Sawaki M, Hattori M, Yoshimura A, Kotani H, Kataoka A, Sugino K, Horisawa N, Ozaki Y, Endo Y, Nozawa K, Takatsuka D, Iwata H :Impact of adjuvant endocrine therapy on prognosis in small hormone receptor-positive, HER2-negative early breast cancer.Breast Cancer, 28(5):1087-1095, 2021.9
- Itoh N, Murakami H, Ishibana Y, Matsubara Y, Yaguchi T, Kamei K :Challenges in the diagnosis and management of central line-associated blood stream infection due to Exophiala dermatitidis in an adult cancer patient.J Infect Chemother,27(9):1360-1364, 2021.9
- Inoue A, Murata K, Komori T, Takeda T, Fujii M, Yamaguchi T, Yamaguchi T, Masuishi T, Shiota T, Morita S, Suzuki Y, Ito M, Kanemitsu Y, Shiozawa M, Yasui M, Kagawa Y, Sugihara K; Study Group of Appendiceal Neoplasms from the Japan Society of Colorectal Cancer Research Group: Open versus laparoscopic surgery for primary appendiceal tumors: a large multicenter retrospective propensity score-matched cohort study in Japan. Surg Endosc. 35(10):5515-5523, 2021. 10
- Chida K, Kotani D, Masuishi T, Kawakami T, Kawamoto Y, Kato K, Fushiki K, Sawada K, Kumanishi R, Shirasu H, Matsubara Y, Yuki S, Komatsu Y, Yamazaki K, Yoshino T: The Prognostic Impact of KRAS G12C Mutation in Patients with Metastatic Colorectal Cancer: A Multicenter Retrospective Observational Study. Oncologist. 26(10):845-853, 2021. 10
- Satoh T, Kato K, Ura T, Hamamoto Y, Kojima T, Tsushima T, Hironaka S, Hara H, Iwasa S, Muro K, Yasui H, Minashi K, Yamaguchi K, Ohtsu A, Doki Y, Matsumura Y, Kitagawa Y: Five-year follow-up of nivolumab treatment in Japanese patients with esophageal squamous-cell carcinoma (ATTRACTION-1/ONO-4538-07). Esophagus, 18(4):835-843, 2021. 10
- Taniguchi H, Yoshino T, Yamaguchi K, Yamazaki K, Nixon AB, Tabernero J, Van Cutsem E, Robling KR, Abada PB, Hozak RR, Siegel R, Fill JA, Wijayawardana S, Walgren RA, Giles B, Jones A, Pitts KR, Drove N, Muro K: Clinical development and evaluation of a VEGF-D assay in plasma from patients with metastatic colorectal cancer in the RAISE study. Curr Med Res Opin, 37(10):1769-1778, 2021. 10
- Catenacci DV, Chao J, Muro K, Al-Batran SE, Klempner SJ, Wainberg ZA, Shah MA, Rha SY, Ohtsu A, Liepa AM, Knoderer H, Chatterjee A, Van Cutsem E: Toward a Treatment Sequencing Strategy: A Systematic Review of Treatment Regimens in Advanced Gastric Cancer/Gastroesophageal Junction Adenocarcinoma. Oncologist, 26(10):e1704-e1729, 2021. 10
- Nakazawa T, Narita Y, Kumanishi R, Ogata T, Matsubara Y, Nozawa K, Kato K, Honda K, Masuishi T, Bando H, Kadowaki S, Ando M, Hara K, Tajika M, Muro K: Second-line Chemotherapy for Previously Treated Metastatic Small Bowel Adenocarcinoma: A Retrospective Analysis. Anticancer Res, 41(10):5147-5155, 2021. 10
- Kataoka K, Yamada T, Taniguchi H, Ikeda M, Yamazaki K, Kanemitsu Y. A ctDNA-driven multidisciplinary treatment strategy for resectable colorectal cancer -what surgical oncologists should know. Eur J Surg Oncol. S0748-7983(21)00752-6, 2021. 10
- Jogo T, Nakamura Y, Shitara K, Bando H, Yasui H, Esaki T, Terazawa T, Satoh T, Shinozaki E, Nishina T, Sunakawa Y, Komatsu Y, Hara H, Oki E, Matsuhashi N, Ohta T, Kato T, Ohtsubo K, Kawakami T, Okano N, Yamamoto Y, Yamada T, Tsuji A, Odegaard JI, Taniguchi H, Doi T, Fujii S, Yoshino T. Circulating Tumor DNA Analysis Detects FGFR2 Amplification and Concurrent Genomic Alterations Associated with FGFR Inhibitor Efficacy in Advanced Gastric Cancer. Clin Cancer Res. 27(20):5619-5627, 2021. 10
- Yamamoto S, Nagashima K, Kawakami T, Mitani S, Komoda M, Tsuji Y, Izawa N, Kawakami K, Yamamoto Y, Makiyama A, Yamazaki K, Masuishi T, Esaki T, Nakajima TE, Okuda H, Moriwaki T, Boku N: Second-line chemotherapy after early disease progression during first-line chemotherapy containing bevacizumab for patients with metastatic colorectal cancer. BMC Cancer. 21(1):1159, 2021.10
- Nozawa K, Yoshimura A, Iwata H :Adjuvant Olaparib in BRCA-Mutated Breast Cancer.N Engl J Med, 385(15):1439, 2021.10
- Taniguchi H, Masuishi T, Kawazoe A, Muro K, Kadowaki S, Bando H, Iino S, Kageyama R, Yoshino T: Phase I study of napabucasin in combination with FOLFIRI + bevacizumab in Japanese patients with metastatic colorectal cancer. Int J Clin Oncol, 26(11):2017-2024, 2021. 11
- Nakamura Y, Fujisawa T, Taniguchi H, Bando H, Okamoto W, Tsuchihara K, Yoshino T, Ohtsu A: SCRUM-Japan GI-SCREEN and MONSTAR-SCREEN: Path to the realization of biomarker-guided precision oncology in advanced solid tumors. Cancer Sci. 112(11):4425-4432, 2021. 11
- Nakamura Y, Okamoto W, Kato T, Esaki T, Kato K, Komatsu Y, Yuki S, Masuishi T, Nishina T, Ebi H, Sawada K, Taniguchi H, Fuse N, Nomura S, Fukui M, Matsuda S, Sakamoto Y, Uchigata H, Kitajima K, Kuramoto N, Asakawa T, Olsen S, Odegaard JI, Sato A, Fujii S, Ohtsu A, Yoshino T: Circulating tumor DNA-guided treatment with pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer: a phase 2 trial. Nat Med. 27(11):1899-1903, 2021. 11
- Iwasa S, Muro K, Morita S, Park YS, Nakamura M, Kotaka M, Nishina T, Matsuoka H, Ahn JB, Lee KW, Hong YS, Han SW, Cho SH, Zhang DS, Fang WJ, Bai L, Yuan XL, Yuan Y, Yamada Y, Sakamoto J, Kim TW: Impact of UGT1A1 genotype on the efficacy and safety of irinotecan-based chemotherapy in metastatic colorectal cancer. Cancer Sci, 112(11):4669-4678, 2021. 11
- Van Cutsem E, Amonkar M, Fuchs CS, Alsina M, Özgüroğlu M, Bang YJ, Chung HC, Muro K, Goekkurt E, Benson AB 3rd, Sun W, Wainberg ZA, Norquist JM, Chen X, Shih CS, Shitara K:Health-related quality of life in advanced gastric/gastroesophageal junction cancer with second-line pembrolizumab in KEYNOTE-061. Gastric Cancer, 24(6):1330-1340, 2021. 11
- Okuyama H, Kagawa Y, Masuishi T, Mishima S, Shirasu H, Ando K, Yuki S, Muro K, Yoshino T, Yamazaki K, Oki E, Komatsu Y, Tsuji A: Infusion-related reaction to ramucirumab plus FOLFIRI in patients with advanced colorectal cancer. Int J Clin Oncol, 26(11):2025-2028, 2021. 11
- Van Cutsem E, Amonkar M, Fuchs CS, Alsina M, Özgüroğlu M, Bang YJ, Chung HC, Muro K, Goekkurt E, Benson AB 3rd, Sun W, Wainberg ZA, Norquist JM, Chen X, Shih CS, Shitara K: Correction to: Health-related quality of life in advanced gastric/gastroesophageal junction cancer with second-line pembrolizumab in KEYNOTE-061. Gastric Cancer, 24(6):1341. doi: 10.1007/s10120-021-01248-8, 2021. 11
- Arai H, Inoue E, Yamaguchi K, Boku N, Hara H, Nishina T, Tsuda M, Shitara K, Shinozaki K, Nakamura S, Hyodo I, Muro K, Sasako M, Terashima M, Nakajima TE: Clinical implications of using both fluoropyrimidine and paclitaxel in patients with severe peritoneal metastasis of gastric cancer: A post hoc study of JCOG1108/WJOG7312G. Cancer Med, 10(21):7673-7682, 2021. 11
- Muro K: [Lower G. I./Colon and Rectum Cancer Frontiers of Perioperative Treatment for Colorectal Cancer]. Gan To Kagaku Ryoho, 48(11):1335-1336, 2021. 11
- Mori M, Honda K, Tsubouchi H, Sakata J, Kato S, Suzuki S: Neoadjuvant chemotherapy for Ewing's sarcoma family tumors of the uterine cervix: A case report. Gynecol Oncol Rep, 38: 100895, 2021. 11
- Kotani D, Yoshino T, Kotaka M, Kawazoe A, Masuishi T, Taniguchi H, Yamazaki K, Yamanaka T, Oki E, Muro K, Komatsu Y, Bando H, Satake H, Kato T, Tsuji A. Combination therapy of capecitabine, irinotecan, oxaliplatin, and bevacizumab as a first-line treatment for metastatic colorectal cancer: Safety lead-in results from the QUATTRO-II study. Invest New Drugs, 39(6):1649-1655, 2021. 12
- Cao Y, Qin S, Luo S, Li Z, Cheng Y, Fan Y, Sun Y, Yin X, Yuan X, Li W, Liu T, Hsu CH, Lin X, Kim SB, Kojima T, Zhang J, Lee SH, Bai Y, Muro K, Doi T, Bai C, Gu K, Pan HM, Bai L, Yang JW, Cui Y, Lu W, Chen J: Pembrolizumab versus chemotherapy for patients with esophageal squamous cell carcinoma enrolled in the randomized KEYNOTE-181 trial in Asia. ESMO Open, 7(1):100341, 2021. 12
- Narita Y, Sasaki E, Masuishi T, Taniguchi H, Kadowaki S, Ito S, Yatabe Y, Muro K: PD-L1 immunohistochemistry comparison of 22C3 and 28-8 assays for gastric cancer.J Gastrointest Oncol,12(6):2696-2705, 2021.12
- Takahashi Y, Sunakawa Y, Inoue E, Kawabata R, Ishiguro A, Kito Y, Akamaru Y, Takahashi M, Yabusaki H, Matsuyama J, Makiyama A, Tsuda M, Suzuki T, Yasui H, Matoba R, Kawakami H, Nakajima TE, Muro K, Ichikawa W, Fujii M: Real-world effectiveness of nivolumab in advanced gastric cancer: the DELIVER trial (JACCRO GC-08). Gastric Cancerm, 25(1):235-244, 2022.1
- Fuchs CS, Özgüroğlu M, Bang YJ, Di Bartolomeo M, Mandala M, Ryu MH, Fornaro L, Olesinski T, Caglevic C, Chung HC, Muro K, Van Cutsem E, Elme A, Thuss-Patience P, Chau I, Ohtsu A, Bhagia P, Wang A, Shih CS, Shitara K: Pembrolizumab versus paclitaxel for previously treated PD-L1-positive advanced gastric or gastroesophageal junction cancer: 2-year update of the randomized phase 3 KEYNOTE-061 trial. Gastric Cancer, 25(1):197-206, 2022.1
- Kang YK, Morita S, Satoh T, Ryu MH, Chao Y, Kato K, Chung HC, Chen JS, Muro K, Kang WK, Yeh KH, Yoshikawa T, Oh SC, Bai LY, Tamura T, Lee KW, Hamamoto Y, Kim JG, Chin K, Oh DY, Minashi K, Cho JY, Tsuda M, Sameshima H, Chen LT, Boku N: Exploration of predictors of benefit from nivolumab monotherapy for patients with pretreated advanced gastric and gastroesophageal junction cancer: post hoc subanalysis from the ATTRACTION-2 study. Gastric Cancer, 25(1):207-217, 2022.1
- Watanabe M, Toh Y, Ishihara R, Kono K, Matsubara H, Murakami K, Muro K, Numasaki H, Oyama T, Ozawa S, Saeki H, Tanaka K, Tsushima T, Ueno M, Uno T, Yoshio T, Usune S, Takahashi A, Miyata H; Registration Committee for Esophageal Cancer of the Japan Esophageal Society: Comprehensive registry of esophageal cancer in Japan, 20Esophagus, 19(1):1-26, 2022.1
- Muro K, Kojima T, Moriwaki T, Kato K, Nagashima F, Kawakami H, Ishihara R, Ogata T, Satoh T, Iwakami K, Han S, Yatsuzuka N, Takami T, Bhagia P, Doi T: Second-line pembrolizumab versus chemotherapy in Japanese patients with advanced esophageal cancer: subgroup analysis from KEYNOTE-181. Esophagus, 19(1):137-145, 2022.1
- Yamaguchi K, Boku N, Muro K, Yoshida K, Baba H, Tanaka S, Akamatsu A, Sano T: Real-world safety and effectiveness of nivolumab in Japanese patients with unresectable advanced or recurrent gastric/gastroesophageal junction cancer that has progressed after chemotherapy: a postmarketing surveillance study. Gastric Cancer, 25(1):245-253, 2022.1
- Fakih MG, Kopetz S, Kuboki Y, Kim TW, Munster PN, Krauss JC, Falchook GS, Han SW, Heinemann V, Muro K, Strickler JH, Hong DS, Denlinger CS, Girotto G, Lee MA, Henary H, Tran Q, Park JK, Ngarmchamnanrith G, Prenen H, Price TJ: Sotorasib for previously treated colorectal cancers with KRASG12C mutation (CodeBreaK100): a prespecified analysis of a single-arm, phase 2 trial. Lancet Oncol. 23(1):115-124, 2022.1
- Yoshikawa AK, Yamaguchi K, Muro K, Takashima A, Ichimura T, Sakai D, Kadowaki , Chin K, Kudo T, Mitani S, Kitano S, Thai D, Zavodovskaya M, Liu J, Boku N, Satoh T: Safety and tolerability of andecaliximab as monotherapy and in combination with an anti-PD-1 antibody in Japanese patients with gastric or gastroesophageal junction adenocarcinoma: a phase 1b study. J Immunother Cancer.10(1):e003518, 2022.1
- Takeyama H, Murata K, Takeda T, Fujii M, Kagawa Y, Kawachi H, Yamaguchi T, Noura S, Masuishi T, Inoue A, Takii Y, Suto T, Sakamoto K, Tei M, Kishimoto M, Yao T, Sugihara K (study group of appendiceal neoplasms in Japan Society of Colorectal Cancer Research Group): Clinical Significance of Lymph Node Dissection and Lymph Node Metastasis in Primary Appendiceal Tumor Patients After Curative Resection: a Retrospective Multicenter Cohort Study, J Gastrointest Surg. 26(1):128-140, 2022.1
- Onishi S, Tajika M, Tanaka T, Yamada K, Kamiya T, Abe T, Higaki E, Fujieda H, Nagao T, Inaba Y, Muro K, Shimizu M, Niwa Y: Effect of Body Composition Change during Neoadjuvant Chemotherapy for Esophageal Squamous Cell Carcinoma. J Clin Med. 11(3):508, 2022.1
- Kataoka K, Yamada T, Taniguchi H, Ikeda M, Yamazaki K, Kanemitsu Y: A ctDNA-driven multidisciplinary treatment strategy for resectable colorectal cancer -what surgical oncologists should know.Eur J Surg Oncol, 48(1):12022.1
- Haddad RI, Seiwert TY, Chow LQM, Gupta S, Weiss J, Gluck I, Eder JP, Burtness B, Tahara M, Keam B, Kang H, Muro K, Albright A, Mogg R, Ayers M, Huang L, Lunceford J, Cristescu R, Cheng J, Mehra R: Influence of tumor mutational burden, inflammatory gene expression profile, and PD-L1 expression on response to pembrolizumab in head and neck squamous cell carcinoma. J Immunother Cancer. 10(2):e003026, 2022 .2
- Ogata T, Fujita Y, Muro K: Dramatic Response to Trastuzumab Deruxtecan Rechallenge in a Patient with HER2-Positive Gastric Cancer: A Case Report. Am J Case Rep, 4;23:e935600.2022.3
- Horisawa N, Adachi Y, Takatsuka D, Nozawa K, Endo Y, Ozaki Y, Sugino K, Kataoka A, Kotani H, Yoshimura A, Hattori M, Sawaki M, Iwata H :The frequency of low HER2 expression in breast cancer and a comparison of prognosis between patients with HER2-low and HER2-negative breast cancer by HR status.Breast Cancer, 29(2):234-241,2022 .3
- Kang YK, Chen LT, Ryu MH, Oh DY, Oh SC, Chung HC, Lee KW, Omori T, Shitara K, Sakuramoto S, Chung IJ, Yamaguchi K, Kato K, Sym SJ, Kadowaki S, Tsuji K, Chen JS, Bai LY, Oh SY, Choda Y, Yasui H, Takeuchi K, Hirashima Y, Hagihara S, Boku N: Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 23(2):234-247,2022.2
和文論文
- 安藤正志:総論 がんの診断・治療に関するインフォームド・コンセント.標準的医療説明 初版,医学書院:36-41, 2021.4月
- 門脇 重憲:大腸癌、肛門がん.新臨床腫瘍学 改訂第6版,南江堂:429-436,2021.5月
- 杉山圭司、成田有季哉、室 圭:胃がん治療state-of-the-art(切除不能転移性). 腫瘍内科 第27巻第6号,科学評論社:607-614,2021. 6月
- 松原裕樹、坂東英明:MSS消化管がんに対する免疫チェックポイント阻害薬の臨床開発と展望. 腫瘍内科 第27巻第6号,科学評論社:675-682,2021. 6月
- 安藤正志:52 希少がん.新臨床腫瘍学 改訂第6版,南江堂:721-725,2021.6月
- 安藤正志:希少がん診療の現状.腫瘍内科第28巻第1号,化学評論社:2-6,2021.7月
- 成田有起哉、中澤泰子:切除不能・再発十二指腸癌を含む小腸癌にMSI検査,HER2検査,RAS遺伝子検査は推奨されるか?.十二指腸癌診療ガイドライン2021年版, 金原出版:69-73,2021.8月
- 室 圭、能澤一樹:切除不能・再発十二指腸癌を含む小腸癌に免疫チェックポイント阻害薬は推奨されるか?.十二指腸癌診療ガイドライン2021年版, 金原出版:78-79,2021.8月
- 室 圭(座談会):消化器病学サイエンス.vol.5 no.3 2021,先端医学社:1-10,2021.9月
- 能澤一樹:術後CDK4/6阻害薬.腫瘍内科 vol.28 No3, 科学評論社:312-317,2021.9月
- 中田晃暢、舛石俊樹、室 圭:切除不能進行再発大腸癌に対する抗がん化学療法.臨床現場で役立つ最新の治療CYRRENT THERAPY,ライフメディコム:75-82,2021.10月
- 松原裕樹、室 圭:胃癌治療ガイドライン2021.消化器・肝臓内科第10巻第2号,科学評論社:275-284,2021.8月
- 児玉紘幸:切除不能進行・再発大腸癌に対する後方ラインにおけるレゴラフェニブ療法とS-1+ベバシズマブ併用療法の無作為化比較第Ⅱ相試験(OGSG1301).癌と化学療法Vol.48 NO.10,癌と化学療法:1241-1246,2021.10月
- 熊西亮介、門脇重憲、室 圭:免疫チェックポイント阻害剤による消化管がんの治療.Cefiro : 最新医療情報誌 34巻,メデカ ジャパン ラボラトリー:10-18,2021.10月
- 門脇 重憲:頭頸部がんにおけるリキッドバイオプシーの現状と未来.Precision Medicine 4巻 12号,北隆館:34-38,2021.10月
- 門脇 重憲:切除不能進行再発大腸がんの薬物療法.日本IVR学会雑誌 36巻 1号,メディカル教育研究社:64-68,2021.10月
- 門脇 重憲、橋本直弥:眼障害.がん薬物療法の支持療法マニュアル 改訂第2版,南江堂:166-172,2021.10月
- 門脇 重憲:1.副反応の対策 [3]腎障害(シスプラチンの減量規準を含む).頭頸部がん薬物療法ハンドブック 改訂3版,中外医学社,2021.11月
- 松原裕樹、室 圭:大腸癌の薬物療法.専門医のための消化器病学第3版,医学書院:270-273,2021.11月
- 中田晃暢、成田有季哉、室 圭:切除不能進行・再発胃癌に対する最新の化学療法.消化器クリニカルアップデート 第3巻第1号,医学図書出版:16-24,2021.11月
- 室 圭:大腸癌周術期治療の最前線(Current Organ Topics)総括.癌と化学療法第48巻第11号,癌と化学療法社:1335,2021.11月
- 室 圭:編集後記.癌と化学療法第48巻第11号,癌と化学療法社:2021.11月
- 室 圭:グレリン様作用薬アナモレリン塩酸塩の位置づけ アナモレリン塩酸塩の副作用への対応.PROGRESS IN MEDICINE 2021 11,ライフ・サイエンス:73-78,2021.11月
- 谷口浩也:がんゲノム医療におけるリキッドバイオプシーの位置づけと最適化.腫瘍内科 vol.29 No1, 科学評論社:7-10,2022.1月
- 能澤一樹:乳がんにおけるゲノム医療.腫瘍内科 vol.29 No.1, 科学評論社:45-50,2022.1月
- 門脇重憲:頭頚部がん.腫瘍内科 vol.29 No.1, 科学評論社:51-57,2022.1月
- 室 圭:アナモレリン塩酸塩錠.日本病院薬剤師会雑誌 vol.58 No1 2022,日本病院薬剤師会:105-108,2022
- 谷口浩也:リキッドバイオプシーを用いた個別化医療:CIRCULATE-Japan.がん分子標的治療 第19巻第2号,メディカルレビュー社:101-103,2022.1月
- 門脇重憲:頭頚部がん,食道がん,胃がん.腫瘍内科 vol.29 No.2, 科学評論社:232-238,2022.2月
- 谷口浩也:疾患レジストリリアルワールドデータの医薬品医療機器承認申請への活用の実際.腫瘍内科 vol.29 No.3, 科学評論社:354-358,2022.3月
- 室 圭:胃癌悪物療法の考え方:総論.日本臨牀80巻増刊号3臨床胃癌学-基礎・臨床の最新動向,日本臨牀社:251-257,2022.3月
- 緒方貴次:胃癌の二次治療・三次治療.日本臨牀80巻増刊号3臨床胃癌学-基礎・臨床の最新動向,日本臨牀社:268-274,2022.3月
- 児玉紘幸:非治癒因子を有する高度進行胃癌.日本臨牀80巻増刊号3臨床胃癌学-基礎・臨床の最新動向,日本臨牀社:288-292,2022.3月
- 松原裕樹:胃癌における術後補助化学療法.日本臨牀80巻増刊号3臨床胃癌学-基礎・臨床の最新動向,日本臨牀社:298-303,2022.3月
- 室 圭:EGFR阻害薬.がんがみえる第1版,メディックメディア:138-141,2022.2月
- 安藤正志:悪性胸水 悪性腹水 原発不明がん.がんがみえる第1版,メディックメディア:594-601,2022.2月
- 門脇重憲:腹膜播種を有する胃癌に対して免疫チェックポイント阻害剤は全身化学治療として推奨されるか?.腹膜播種診療ガイドライン2021年版,金原出版:34-35,2021
- 門脇重憲:10.その他の臓器横断的バイオマーカー.成人・小児進行固形がんにおける臓器横断的ゲノム診療のガイドライン第3版,金原出版:79-95,2022年2月
学会発表
- 室 圭:消化管癌に対する免疫チェックポイント阻害剤治療の位置づけと今後の展望.第107回日本消化器病学会総会,ワークショップ(基調講演),東京(ハイブリット開催),2021.4月WS10-1
- Masuishi T, Taniguchi H, Kotani D, Bando H, Satoh T, Esaki T, Komatsu Y, Sunakawa Y, Nishina T, Shinozaki E, Nishida N, Komoda M, Yuki S, Izawa N, Sharma G,Skrzypczak S, Schultz E, Kingsford C, Sato A, Yoshino T:Discovery of a potential predictive marker for eribulin treatment and noveltarget genes in BRAF V600E mutant metastatic colorectal cancer using an AI-driven RNA-seq analysis platform: Translational research of the BRAVERY study(EPOC1701). ASCO 2021, publication only, Virtual, 2021. 6月
- Bando H, Nakamura Y, Taniguchi H, Shiozawa M, Yasui H, Esaki T, Ohta T, Denda T, Satoh T, Yamazaki K, Sunakawa Y, Kato T, Goto M, Yuki S, Nishina T, Oki E, Shinozaki E, Matsuhashi N, Hata M, Yoshino T: Impact of a metastatic site on circulating tumor DNA (ctDNA) analysis in patients(pts) with metastatic colorectal cancer (mCRC). ASCO 2021, Poster Session, Virtual, 2021. 6月
- Matsubara Y, Toriyama K, Kadowaki S, Ogata T, Nakazawa T, Kato K, Nozawa K, Narita Y, Honda K, Masuishi T, Bando H, Ando M, Tajika M, Hosoda W, Muro K: Impact of PD-L1 combinedpositive score (CPS) on clinical response tonivolumab in patients with advanced esophageal squamous cell carcinoma. ASCO 2021, publication only, Virtual, 2021. 6月
- Janjigian YY, Cutsem EV, Muro K, Wainberg ZA, Al-Batran S-E, Hyung WJ, Molena D, Evans B, Dario Ruscica D, Scott H. Robbins SH, Alejandra Negro A, Josep Tabernero J: MATTERHORN: Efficacy and safety of neoadjuvant-adjuvant durvalumab andFLOT chemotherapy in resectable gastric and gastroesophageal junction cancer—A randomized, double-blind, placebo-controlled, phase 3 study. ASCO 2021, Poster Session, Virtual, 2021. 6月
- Takiguchi T, Shitara K, Takiguchi N, Ito S, Kochi M, Horinouchi H, Kinoshita T, Muro K, Yoshikawa T, Hasegawa H, Nishikawa H, Kodera Y: Neoadjuvant nivolumab monotherapy in patients with resectable gastric cancer:Preliminary results from a multicenter study. ASCO 2021, Poster Session , Virtual, 2021. 6月
- Ciardiello F, Bang Y-J, Bendell JC, Cervantes A, Dvorkin M, D.Lopez C, Metges J-P, Sanchez A, Calvo M, Strickland A, Kannourakis G, Muro K, Kawakami H, Wei J, Borg C, Song M, Zhang K, Zhang M, Shen L: PARALLEL 303: Phase 2 randomized study of pamiparib vs placebo asmaintenance therapy in patients (pts) with inoperable locally advanced ormetastatic gastric cancer that responded to platinum-based fi rst-line (1L)chemotherapy. ASCO 2021, Poster Session , Virtual, 2021. 6月
- Okamoto W, Nakamura Y, Kato T, Esaki T, Komoda M, Kato K, Komatsu Y, Masuishi T, Nishina T, Sawada K, Taniguchi H, Fuse N, Nomura S, Fukui M, Olsen SR, Odegaard JI, Sato A, Fujii S, Ohtsu A, Yoshino T: Pertuzumab plus trastuzumab and real-world standard of care (SOC) forpatients (pts) with treatment refractory metastatic colorectal cancer (mCRC) with HER2 (ERBB2) amplifi cation (amp) confi rmed by tumor tissue or ctDNA analysis (TRIUMPH, EPOC1602). ASCO 2021, Poster Session , Virtual, 2021. 6月
- Yu Nishina T, Yasui H, Ohta T, Takahashi N, Satake H, Kanazawa A, Goto M, Bando H, Taniguchi T, Okugawa Y, Yamazaki K, Ebi H, Abe Y, Nomura S, Asano C, Yoshino T: Profiling plasma angiogenesis factors after use of biologics in metastaticcolorectal cancer (mCRC): Update results from GI-SCREEN CRC Ukit study. ASCO 2021, Poster Session , Virtual, 2021. 6月
- Kuboki Y, Terazawa T, Masuishi T, Nakamura M, Watanabe J, Ojima H, Shinohara Y, Kotaka M, Hara H, Ohta T, Oki E, Sunakawa Y, Ishihara S, Taniguchi H, Eguchi Nakajima T,Morita S, Shirao K, Yoshino T: The TRUSTY study: A randomized phase 2/3 study of trifl uridine/tipiracil plusbevacizumab versus irinotecan and fl uoropyrimidine plus bevacizumab assecond-line treatment in patients with metastatic colorectal cancer. ASCO 2021, Poster Session , Virtual, 2021. 6月
- Bando H, Kinoshita I, Modi S, Tsurutani J, Bang Y-J, Iwata H, Sato Y, Nakatani S, Lee CC, Sugihara M, Okuda Y, Takahashi S: Trastuzumab deruxtecan (T-DXd) in patients with human epidermal growthfactor receptor 2 (HER2)-expressing salivary duct carcinoma: Subgroup analysisof two phase 1 studies. ASCO 2021, Poster Session , Virtual, 2021. 6月
- Yoshino T, Bartolomeo MD, Raghav KPS, Masuishi T, Loupakis F, Kawakami H, Yamaguchi K, Nishina T, Wainberg ZA, Elez E, Rodriguez J, Fakih M, Ciardiello F, Saxena K, Kobayashi K, Bako E, Okuda Y, Meinhardt G, Grothey A, Siena S: Trastuzumab deruxtecan (T-DXd; DS-8201) in patients (pts) with HER2-expressing metastatic colorectal cancer (mCRC): Final results from a phase 2,multicenter, open-label study (DESTINY-CRC01). ASCO 2021, Oral Abstract Session , Virtual, 2021. 6月
- 藤澤孝夫、門脇重憲、佐竹悠良、洞澤智至、倉本尚美、坂本泰理、中村能章、谷口浩也、吉野孝之、岡野晋:SCRUM-Japan MONSTAR-SCREENにおける頭頸部がん血漿循環腫瘍DNA(ctDNA)遺伝子異常の検討.第45回日本頭頚部癌学会総会,口演,千葉(ハイブリット開催)Virtual, 2021.6月O-064
- Muro K (Chair): Session IV: Presentation of Selected Non-Colorectal Cancer Abstracts. ESMO-GI 2021, Short Oral Session, Virtual, 2021. 7月
- Muro K (Speaker):Discussant: LBA-4, Selected Oral Abstract, LBAESMO-GI 2021, Discussion, Virtual, 2021. 7月
- Muro K (Panelist): Cases on Management Strategy in Esophageal and GE Junction Cancer. ESMO-GI 2021, Session X: Esophageal and Gastric Cancers, Virtual, 2021. 7月